Abstract:
A zero-linked polymeric hemoglobin (OxyVita Hb) has been developed for application as an acellular therapeutic hemoglobin-based-oxygen-carrier (HBOC). For effective and safe oxygen binding, transport and delivery, an HBOC must meet essential molecular requirements related to its structural integrity and redox stability. OxyVita™ is a super polymer possessing an average M.wt. of 17 × 106 Da. Structural integrity was determined by unfolding studies of OxyVita™ in the presence of increasing concentrations of urea. The unfolding midpoints (D1/2) of different preparations of OxyVita™ (solution and powder forms) were compared to Lumbricus Hb (LtHb) and Arenicola Hb (ArHb), natural acellular polymeric hemoglobins, which are serving as models for an effective and safe acellular HBOC. Reduction studies of OxyVita Hb using endogenous reducing agents were also investigated. Results from these studies indicate that: 1) OxyVita Hb exhibits greater resistance to conformational change than either LtHb or ArHb in the reduced (oxyHb) state; and 2) the reduction of met OxyVita Hb to oxyHb occurs slowly in the presence of either ascorbic acid (70% reduction in 560 min.) or β-NADH (40% reduction in 90 min.). These studies provide consistent evidence that OxyVita Hb possesses physiochemical properties that exhibit structural integrity and redox behavior necessary for functioning as an effective and safe HBOC within clinical applications. These results are in agreement with observations made by other investigators as to the reduction in heme-loss of OxyVita Hb, essential for the reversible binding/release of molecular oxygen within the circulatory system.
INTRODUCTION
Natural acellular polymeric hemoglobins (Hb) provide oxygen transport and delivery within many terrestrial and marine invertebrate organisms. It has been our premise that these natural acellular Hbs can serve as models for the development of therapeutic hemoglobin-based oxygen carriers (HBOC). An understanding of how other organisms utilize acellular oxygen carriers and maintain their structural integrity and redox stability within their circulatory systems is vital for the design of a safe and effective red cell substitute [Citation1]. Recently, a zero-linked polymeric hemoglobin (OxyVita™), a new generation HBOC, has been developed for application as an acellular therapeutic hemoglobin-based-oxygen-carrier [Citation2,Citation3]. For effective and safe oxygen binding, transport and delivery, an HBOC must meet essential molecular requirements related to structural integrity and redox stability. OxyVita Hb, a super polymer, has an average molecular weight of 17 MDa and a viscosity at 6g% comparable to human plasma [Citation4]. Initial animal studies resulted in no observed vascular extravasation as indicated by OxyVita Hb’s absence in the renal hilar lymph after exchange transfusion in animals [Citation5]. No evidence of this protein within the urine was also determined [Citation6].
It is vital that an understanding and appropriate determination of the physicochemical characteristics of this new polymeric HBOC be available for all potential users and proposed applications for this new generation HBOC. The recent work of Jia and Alayash [Citation7], which has focused on this zero-linked polymeric hemoglobin, clearly provides through a series of specific studies the beginnings of a transparent evaluation of this unique HBOC as a potentially viable HBOC.
The present study extends this biochemical and biophysical evaluation of the zero-linked polymeric hemoglobin, OxyVita Hb, through a series of unfolding studies that provide evidence regarding the structural integrity and reduced heme loss within this protein under significant solution perturbation. The presence of the heme moiety within the folded globin chain is essential for both functional as well as overall thermodynamic stabilization to the hemoglobin molecule, regardless of its hierarchical structure. The strength of this heme linkage to the globin chains and inherent conformational stability is linked to the redox state of the heme-iron.
In addition, solution redox characteristics of this HBOC are presented that support human plasma’s role in providing protection from rapid autoxidation and maintenance of the reduced state of the heme-Fe+2 necessary for the reversible binding-release of molecular oxygen in vivo.
MATERIALS AND METHODS
Hemoglobins
Zero-Linked Bovine Hemoglobin (OxyVita™) was developed and made available for these studies by OxyVita, Inc. (New Windsor, NY). This polymeric hemoglobin is generated by the formation of pseudopepetide bonds between carboxyl groups and amino groups of the surface of stroma free bovine Hb molecules using a zero-linked activation process [Citation2,Citation3]. Dynamic light scattering [Citation8] was utilized to determine molecular weight distribution and average molecular weight of the zero-linked hemoglobin used in these studies.
Lumbricus terrestris Hb. Live earthworms (10–20 cm) were obtained from Ward’s Biological Company, Rochester, NY. The coelomic fluid of each worm was drained into a cold (∼1°C) 100 mM potassium phosphate buffer, pH 7.0. After initial centrifugation to remove cellular debris, the supernatant was removed and centrifuged at 160,000g for 3 hours at 4°C. The red pellet was redissolved in buffer and the ultracentrifugation step was repeated. This red pellet was redissolved in a minimum amount of buffer to maintain a high stock concentration of the protein. Purified hemoglobin was used immediately or stored in pellet form under liquid nitrogen until required for use. Storage under liquid nitrogen prevents autoxidation. No changes in the properties of this hemoglobin were evident with these stored preparations over at least one year as determined by spectroscopic and light scattering measurements [Citation9].
Arenicola marina Hb. Live lugworms were obtained from the Marine Resource Center at the Marine Biological Laboratory in Woods Hole, MA. A similar method of isolation and purification was employed as described above for the earthworm hemoglobin.
Structural Stability: Isothermal Unfolding Studies
The isothermal unfolding of each of the acelluar hemoglobins (OxyVita Hb, LtHb, ArHb) was obtained at 37°C. Urea solutions of 1 to 9.5 M were prepared by volumetric dilution using a 10 M stock solution. For absorbance determinations, hemoglobin solutions were prepared by dilution in 5 ml volumetric flasks from appropriate stock solutions of each acellular hemoglobin. All protein solutions contained 50 mM HEPES, pH 7.0. Spectral measurements in the Soret region (350–450 nm) were recorded on a Shimadzu 160 recording spectrophotometer after 30 minutes of equilibration at 37°C. The unfolding of each hemoglobin as a function of increasing urea concentration was followed by changes in Soret absorbance maximum, assuming a molar extinction of 1.25 × 105 (10). Hemoglobin concentrations varied between 10−5 to 10−6 M in heme.
Reduction Studies
The rate and extent of each acellular hemoglobin reduction reaction was carried out at 20°C in 50 mM HEPES, pH 7.0. Each hemoglobin was completely oxidized (100% metHb) by reacting each sample with an excess of K3Fe(CN)6. Each oxidized sample was eluted on a Sephadex G-25 column to remove the unreacted potassium ferricyanide. The reduction reactions were initiated by the direct addition of glutathione, ascorbic acid, and β-NADH to give the specified concentration of reducing agent. Using direct absorbance analysis, spectra of the reduction process were recorded in overlay mode within the visible region (490–700 nm) as a function of time. The rate and extent of each hemoglobin reduction was determined either by the increase in the A576/A540 ratio or by the decrease in the charge-transfer band at 630 nm [Citation11].
RESULTS AND DISCUSSION
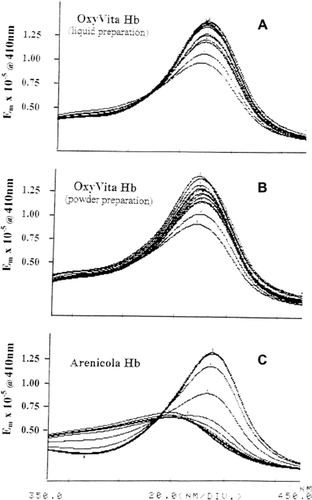
The extent of acellular hemoglobin unfolding and associated heme exposure within the globin in the presence of increasing concentrations of the solvent purturbant urea is presented in and . The Soret spectral region (350–450 nm) was used to determine the extent of hemoglobin unfolding due to its sensitivity to alterations within the heme environment. When these heme proteins are in the presence of a conformational perturbant such as urea, increasing the concentration of urea leads to the disruption of the many intramolecular interactions responsible for the maintenance of the native structure. illustrates the strong resistance to unfolding by increasing concentrations of urea for the liquid and powder preparations of OxyVita™ compared to the hemoglobin isolated from the marine organism, Arencicola marina. The small alterations of the Soret region (absorbance) spectra for the OxyVita™ preparations (up to 7M urea) are in marked contrast to the spectral behavior of ArHb, wherein extensive unfolding has occurred with significant exposure of the heme moiety occurring by 4M urea concentration. This is reflected in the dramatic decrease in absorbance at 410 nm and a marked blue shift in wavelength maximum to 398 nm for the ArHb.
Figure 1. Isothermal unfolding of acellular hemoglobins (Arenicola Hb, Lumbricus Hb, and OxyVita™, liquid and powder preparations) in the presence of urea at T = 37°C. All solutions were equilibrated for 30 minutes prior to spectral runs.
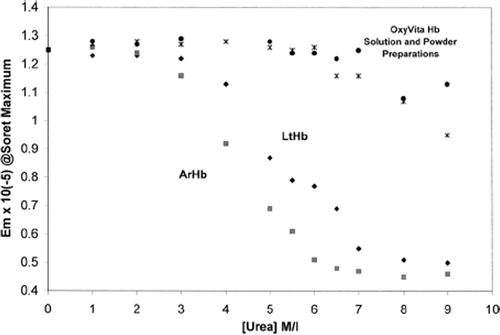
Figure 2. Soret spectra of OxyVita™, liquid preparation (A), powder preparation (B), and Arenicola Hb (C) in urea solution (0-9 M urea) at T = 37°C.
In striking contrast to the unfolding profiles () of the natural acellular hemoglobins from the terrestrial Lumbricus terrestris (LtHb) and the marine Arenicola marina (ArHb), the zero-linked polymeric OxyVita™ (liquid and powder preparations) clearly demonstrates strong resistance to secondary structural unfolding, even at very high urea concentrations. Assuming a two-state model of unfolding, the unfolding midpoints (D1/2) for LtHb and ArHb are 5.4 M and 4.4 M urea, respectively. Extensive resistance to unfolding by either preparation of OxyVita™ is reflected in the much greater unfolding midpoint with D1/2 = 6.8 M urea. Consistent with the significant differences in midpoint unfolding, the free energy of unfolding (ΔGu,°water) in the absence of denaturant was determined to be 4.3 kcal/mol for LtHb, 3.7 kcal/mol for ArHb, and 5.5 kcal/mol for OxyVita™, clearly indicating OxyVita™’s greater resistance to molecular unfolding and the maintenance of its structural integrity under strong perturbation conditions ().
Table 1. Free Energy of Unfolding(ΔG°u) in Absence of Denaturant and Unfolding Midpoint (D1/2) of Acellular Polymeric Hemoglobins T = 37°C
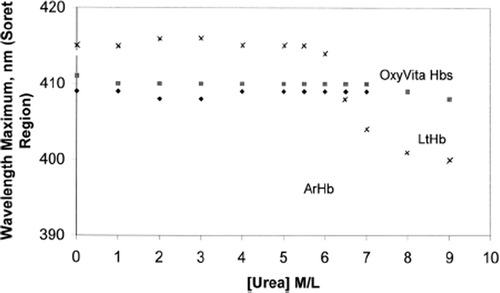
Coupled to these differences in structural unfolding is a change in oxidation state (heme-Fe+2→heme-Fe+3) that is reflected by an observed blue shift in wavelength maximum within the Soret region (350–450 nm). shows a comparison of the changes in the Soret wavelength maximum for LtHb, ArHb, and OxyVita™ (liquid and powder preparations) as a function of increasing urea concentration. In sharp contrast to LtHb and ArHb, wherein the Soret wavelength maximum blue shifts are 413 nm→400 nm and 412 nm→398 nm, respectively, OxyVita™ undergoes little change in the Soret maximum, 410 nm→408 nm. This significant blue shift, indicative of methemoglobin formation (heme-Fe+3), is associated with an increase in the extent of heme exposure within these natural acellular hemglobins upon unfolding. An increase in methemoglobin formation leads to a decrease in oxygen-carrying capacity of any hemoglobin. OxyVita™ resistance to oxidative changes under the strong denaturing conditions in the presence of increasing concentrations of urea is an important characteristic for potential HBOC applications. Minimizing oxidative changes is vital for the introduction of an acellular hemoglobin into the circulatory system wherein increased membrane and tissue interactions occur.
Figure 3. Changes in wavelength maximum within the Soret region for acellular hemoglobins (Arenicola Hb, Lumbricus Hb, and OxyVita™, liquid and powder preparations) in the presence of increasing concentrations of urea at T = 37°C.
Heme-iron oxidation associated with the formation of methemoglobin is often accompanied by hemichrome formation and eventual release of heme-iron responsible for cellular and tissue oxidative events. The recent study of Jia and Alayash [Citation7] demonstrated that the zero-linked OxyVita™ polymer gave evidence of little heme-iron loss, suggesting that its heme stability is related to the compact conformational structure of this large polymer. These results are consistent with the observed resistance to structural unfolding as determined by results presented in this study.
The physiological binding and release of molecular oxygen from any hemoglobin requires that the heme-iron be maintained in the reduced state (heme-Fe+2). Methemoglobin (heme-Fe+3), which can be generated either through autoxidation or the interaction with exogenous oxidizing compounds, is unable to bind oxygen and may lead to hemichrome formation leading to the production of oxidative free radicals in vivo [Citation12–14]. It is essential that any HBOC be maintained in this reduced state to work effectively and safely as an oxygen carrier and delivery system. Lacking the protective array of reducing enzymes normally found within the red blood cell, an HBOC must be capable of being reduced within the circulatory system for sustained functionality.
Although earlier studies indicated that glutathione and β-NADH can reduce met LtHb, a natural acellular hemoglobin, slowly [Citation15], ascorbic acid was rapid and effective in the reduction of this multimeric metHb. Recently, these studies were extended to a marine organism’s acellular hemoglobin (ArHb) and to the zero-linked polymeric OxyVita™. In contrast to HbA, HbXL99α, and Oxyglobin (Biopure product), ArHb and OxyVita™ were reduced significantly by ascorbic acid, 30% within 60 minutes and 70% within 540 minutes, respectively (). The potential role of endogenous ascorbic acid within human plasma is well supported by the recent studies of Cooper et al. [Citation16], wherein a protective strategy involving ascorbate in helping to maintain oxidative stability of infused HBOC has been elucidated.
Table 2. Reduction (heme-Fe+3 heme-Fe+2) of Acellular Hbs by Ascorbic Acid, Glutathione and β-NADH T = 22°C, pH 7.0
In summary, the structural integrity of OxyVita™ as determined in the present studies lends support to: 1) the observed elimination of toxic side-effects associated with previously utilized HBOCs during pre-clinical and clinical studies; and 2) is consistent with the absence of mean arterial pressure (MAP) elevation during use of OxyVita™ in earlier animal investigations [Citation3,Citation5,Citation8].
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Harrington, J.P., Kobayashi, S., Dorman, S.C., Zito, S.L., Hirsch, R.E. (2007). Artificial Cells, Blood Substitute and Immobilized Biotechnology, 35: 53–67.
- Razynska, A., Bucci, E. (1998). Zero-link Polymerization: a New Class of Polymeric Hemoglobins, in Blood Substitutes-Present and Future Perspectives, Tuschida, E., Elsevier Science, Switzerland, 265–279.
- Bucci, E., Kwansa, H., Koehler, R.C., Matheson, B. (2007). Artificial Cells, Blood Substitutes and Immobilized Biotechnology 35: 11–18.
- OXYVITA. Hb preparation based upon patent formulation [US Patent].
- Matheson, B., Razynska, A., Kwansa, H., Bucci, E. (2000). Journal of Laboratory and Clinical Investigation 135: 459–464.
- Bucci, E., Razynska, A., Kwansa, H., Matheson-Urbaitis, B., O’Hearne, J.A., Utalowski, J.A., Koehler, R.C. (1996). Journal of Laboratory and Clinical Medicine 128: 146–153.
- Jia, Y., Alayash A.I. (2009). Biochimica et Biophysica Acta, Proteins and Proteomics 1794: 1234–1242.
- Matheson, B., Kwansa, H.E., Rebel, A., Mito, T., Koehler R.C., Bucci E. (2002). Journal of Applied Physiology 93: 1479–1486.
- Harrington, J.P., Pandofelli E.R., Herskovits, T.T. (1973). Biochimica et Biophysica Acta 328: 71–83.
- DiIori, E. (1981). Methods in Enzymlogy 76: 47–72.
- Harrington, J.P. (1998). Comparative Biochemisty and Physiology B 119B: 305–309.
- Rachmilewitz, E.A. (1974). Seminar in Hematology 11: 441–462.
- Alayash A.I., Cashon, R.E. (1995). Molecular Medicine Today 1: 122–127.
- Faivre, B., Menu, P., Labrude, P., Vigernon, C. (1998). Artificial Cells, Blood Substitute and Immobilized Biotechnology 26: 17–26.
- Harrington, J.P., Gonzalez, Y., Hirsch, R.E. (2000). Artificial Cells, Blood Substitute and Immobilized Biotechnology 28: 477–492.
- Cooper, C.E., Silaghi-Dumitrescu, R., Rukengwa, M., Alayash, A.I., Buehler, P.W. (2008). Biochimica et Biophysica Acta 1784: 1415–1420.
- Dorman, S.C., Kenny, C., Miller, L., Hirsch, R.E., Harrington, J.P. (2002). Artificial Cells, Blood Substitute and Immobilized Biotechnology 30: 43–56.