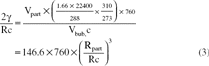Abstract:
Rationale: Earlier work has shown that experimental conditions calling for improved tissue oxygenation could be assisted by i.v. infusion of a dodecafluoropentane emulsion (DDFPe) forming oxygen-transporting microbubbles. Objectives: The present work investigated the effect of DDFPe on hypoxia due to experimental shunts in the pig lung. Methods: Nineteen O2 breathing, anesthetized pigs had glass beads administered into the trachea so as to significantly depress arterial oxygen tension (PaO2). PaO2 was recorded for up to 12 hrs while 0.1 ml/kg DDFPe was administered 1–3 times. Main Results: The animals were divided into two groups based on arterial oxygen saturation (SaO2) after shunt induction, combined with oxygen breathing: the “SaO2 >90% group” (n=6) and the “SaO2 <90% group” (n=13). In the “SaO2 <90% group,” the PaO2 increased stepwise with each infusion from 56.6±2.9 to 88.6±14.6 mmHG (P≤0.001); improvements lasted about 2 hrs after each infusion. Mixed venous oxygenation also increased with the infusions, e.g. (1st infusion) from a PvO2 of 41.4±2.3 to 49.9±4.2 mmHg (P≤0.05) and SvO2 58.0±2.9% (P≤0.01), the venous changes supporting arterial oxygenation. At the same time, arterial CO2 levels fell. Arterial O2 and CO2 levels were paralleled by similar changes in muscle tissue. Pulmonary arterial pressures did not indicate any pulmonary embolization by bubbles. Toxic effects of the treatment were not observed. Conclusion: These results suggest that, on condition of successful toxicity testing, intravascular administration of a DDFPe and oxygen breathing may be beneficial in severe right-to-left shunting in humans.
INTRODUCTION
Earlier work in this laboratory has demonstrated that oxygen breathing, combined with the introduction, by pharmacological means, of micro-bubbles that can carry oxygen in the circulation, was life sustaining in severely anemic rats [Citation1]. The same treatment principle has been employed to enhance tissue oxygenation in hepatoma cells in rats so as to make the cells more radiosensitive [Citation2]. Likewise, we hypothesized that arterial hypoxia due to right-to-left intra-pulmonary shunting could similarly be counteracted.
The concept of physiologically significant oxygen transport by intravascular microbubbles is based on theoretical calculations [Citation3, Citation4] suggesting that microbubbles will, on passage through the lungs, take up oxygen by diffusion and subsequently, by the same physical process, deliver it to the tissues. At the same time, the bubbles would enhance carbon dioxide transport in the opposite direction. A condition for this concept to be practically useful would be that the bubbles contain a poorly soluble gas such as dodecafluoropentane (DDFP) for volume stabilization [Citation3]. The gas in question will slow the collapse of bubbles that occurs in accordance with Laplace’s law, which says that, for a given surface tension, the smaller the radius of a bubble the higher the internal gas pressure and therefore the more rapid the diffusional loss of gas to the surrounding medium.
The preparation used for introducing microbubbles in the circulation in the present study is a 2% w/v DDFP emulsion (DDFPe) in a water phase, which was originally developed as an ultrasound contrast medium (Sonus Pharmaceuticals Inc., Bothell, WA). Upon warming to body temperature, the emulsion particles undergo phase transition (at ∼ 29° C) to gas, i.e. microbubbles are formed, which are small enough to pass through capillaries [Citation5,Citation6] and proceed to equilibrate with other gases in the surrounding plasma and tissues as described above.
It has been calculated that the initial diameter of the microbubbles when only containing DDFP gas is about 2 μm and that it increases to about 3 μm (volume increase 3.38 times) in the lung capillaries by equilibrating with alveolar gas tensions while, after the first passage through the tissue capillaries, it is again reduced to about 2 μm [Citation3]. The bubbles gradually shrink as they continue to circulate and the DDFP gas goes into solution in the plasma from which it is eliminated, mainly via the expired air [Citation7]. The lifespan of such bubbles in the circulation has variously been estimated at between 3 min and 4.4 hrs [Citation8]. However, accurate quantification may be limited by ultrasound-mediated microbubble destruction [Citation9].
The aim of the present investigation was to test whether i.v. administration of DDFPe can ameliorate the hypoxia caused by pulmonary right-to-left shunts in pigs. The adequacy of gas exchange was judged from arterial and venous PO2 and PCO2 levels, tissue PO2 and PCO2, circulatory variables, and from acid-base status.
METHODS
Material
Nineteen pigs of both sexes, weighing 27.8±2.2 kg (mean±SEM), were used for the study. They were housed in the Laboratory Animal Facility, State University of New York at Buffalo. The experiments were approved by the Institutional Animal Care University Committee. The pigs were fasted over night, but had free access to water. They were pre-medicated with Telazol (40 μL/kg i.m.) (Fort Dodge Animal Health, Fort Dodge, IA), given atropine (40 μL/kg i.m.), and anesthetized with sodium pentobarbital (15–20 mg/kg i.v.). The animals were breathing spontaneously and kept at the initial body temperature of 38.7±0.1° C.
Surgical Preparation
The pigs were tracheotomized and breathing air during the surgery and control phases, and breathing oxygen during the experimental phase. The right femoral artery was cannulated for recording of arterial pressure (AP) and heart rate (HR) using a COBE disposable pressure transducer (COBE, Lakewood, CO). A femoral vein was cannulated for continuous i.v. infusion of 1 ml/min of lactated Ringer’s solution and anesthesia-sustaining doses of 2–3 mg/kg/hr of sodium pentobarbital. Arterial blood gas samples were obtained anaerobically with a gas-tight syringe from a catheter in the left femoral artery and measured with a Ciba-Corning Blood Gas System, model 278 (Medfield, MA). The left femoral vein was used for infusing the DDFPe and lactated Ringer’s solution according to a timeline to be described in the following. The right external jugular vein was exposed and cannulated with a balloon-guided catheter, which was introduced into the right cardiac ventricle (RV) and, with the balloon partly inflated, floated with the bloodstream until the tip was positioned in a pulmonary artery, after which the balloon was deflated. The pulmonary arterial pressure (PAP) and right ventricular pressure (RVP) were measured with COBE disposable transducers. A catheter was placed in the right external jugular vein alongside the pulmonary arterial catheter with the tip in the right atrium to measure central venous pressure (CVP) and draw blood samples for determination of mixed venous blood gas. A transcutaneous O2–CO2 Kombi-Sensor (Kontron, Zürich, Switzerland) was placed in a small pouch over exposed abdominal muscle for continuous measurements of muscle PCO2 and PO2 tensions. The temperature of the heating element of the sensors was set at 39.5° C and the sensors calibrated with standard gas mixtures before each experiment. Blood pressures, HR, respiratory frequency (RF), and tissue gas tensions were continuously recorded by a multi-channel recorder (model 2800s, Gould, Lakeview, OH). The instrumentation procedures described above were completed within 1 h in all animals.
Treatment Preparation
The composition of the preparation used in the present experiments has been described as follows: “It consists of an aqueous nano-emulsion of DDFP. This intravenous injectable contains 0.3% weight/volume (w/v) PEG Telomer-B, 0.3% w/v Pluronic P123 and 30% w/v sucrose in addition to 2% DDFP w/v. The mean particle size (intensity weighted) is 232 nM in diameter. Furthermore, 99% of the particles in the formulation are smaller than 626 nm. Thus, the formulation manufactured and stored under pressure does not contain a significant number of particles in the micrometer range” [Citation7].
Experimental Procedure
The sequence of experimental manipulations is shown in . Following surgery, the pigs were allowed to stabilize for approximately 30 min. Control measurements of AP, mean AP (mAP), mean PAP (mPAP), CVP, RF, and HR were recorded over the next 15 min. At least two blood samples were collected and immediately analyzed for arterial and venous PO2, PCO2 and pH.
Figure 1. Arterial oxygen tension (PaO2) (Panel A), carbon dioxide tension (PaCO2) (Panel B) and pH (Panel C) versus time, plotted for pigs with arterial oxygen saturation (SaO2) > 90% pre-treatment (Hsat-Group, upper panels) and pigs with SaO2 < 90% pre-treatment (Lsat-Group, lower panels). Data shown for: air-breathing pre-shunt, during shunt application without and with (subsequent) oxygen breathing, and air-breathing at end of experiments. Number of animals in each time period shown in Panel A. Time and duration of infusions of dodecafluoropentane emulsion (DDFPe) marked in each panel. Values are means±SEM and P-values are shown for data of each time period marked by dashed lines compared with data from the last 10 min before DDFPe treatment.
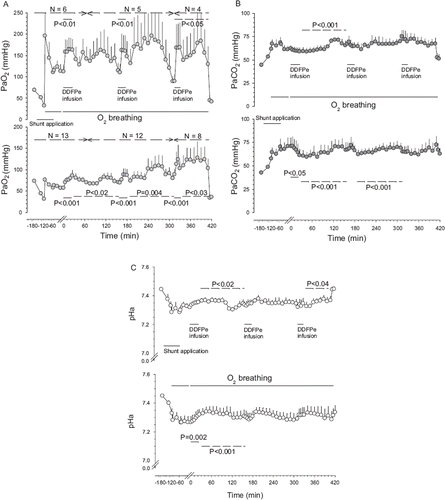
Figure 2. Oxygen tensions (PmO2) (Panel A) and carbon dioxide (PmCO2) (Panel B) tensions versus time in muscle tissue plotted in Hsat-Group and Lsat-Group pigs (for further explanations see legend).
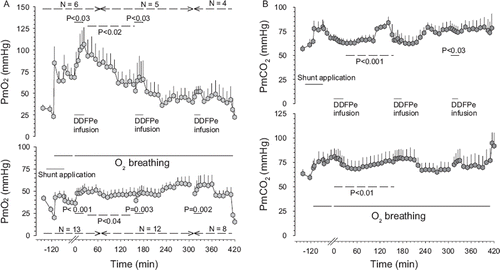
Figure 3. Central venous pressure (Panel A) and right ventricular pressure (Panel B) versus time in Hsat-Group and Lsat-Group pigs (for further explanations see legend).
The circulatory shunts in the lungs of 19 pigs were established essentially as described by Eyal et al. [Citation10]. Thus, blocking of a portion of the airways at roughly the terminal bronchiolar level was achieved by letting the animal inhale steel or glass beads of about 1 mm diameter. The beads were poured into the tracheal tube in several small aliquots for a total dose of about 5 ml. This dosing was guided by development of arterial hypoxia during oxygen breathing as described below. After establishing the shunt, arterial and central venous blood samples were drawn and analyzed. Oxygen breathing started halfway into the bead administration and three new baseline blood gas measurements distributed over 20 min were made and served to determine that a marked depression of the arterial oxygen tension (PaO2) was present before DDFPe infusion was started. The i.v. infusion of DDFPe at a rate of 0.1–0.2 ml/min in parallel with 4 ml/min of lactated Ringer’s solution was then begun. A total of 0.1 ml/kg of DDFPe was given over a 30-min period. Arterial blood samples were taken and analyzed for blood gas tensions every 5 min during the infusion period and every 10 min for the rest of the treatment phase(s).
Of the 19 pigs, two were given only one infusion of the DDFPe, five were given only two and 12 received three infusions of comparable doses of DDFPe.
Regularly, the effect of the first dose of DDFP (a rise in PaO2) started to wear off 2–3 h after the infusion was completed and the blood gas tensions gradually returned to the baseline values. Then, if applicable, the second dose of DDFPe was infused in an identical manner as the first. This was followed by arterial blood sampling every 10 min for the next 3 hrs, again showing increased PaO2. Mixed venous blood samples were drawn less regularly. When PaO2 began to decrease, a third dose of DDFPe was administered, if applicable, in the same way as the first two doses, and PaO2 was followed until a distinct fall was observed. Thereafter, the breathing gas was changed to air and post-experimental physiological recordings performed. Some animals ceased to breathe spontaneously and died shortly after returning to air breathing and the remaining animals were euthanized within 20 min, with 100 mg/kg, i.v. of sodium pentobarbital.
Statistics and Calculations
To test for changes due to the treatment, one-way repeated measure analysis of variance (RM ANOVA), or Friedman repeated measures of variances on ranks (RM ANOVA on ranks) were used. To test differences between the two groups (see Results), Paired t-tests or Wilcoxon signed rank test were used. Changes between time periods were tested with t-test or Mann-Whitney rank sum test. The choice of test was determined by the distribution of the data in each test situation. All data are given as mean ± SEM. All P-values ≤0.05 were considered significant. All blood gas tensions and pH, as well as tissue gas tensions are reported at 37° C, and all data up to the first treatment are derived from measurements in all 19 pigs and presented as such in the running text.
The calculation of hemoglobin oxygen saturation was performed in accordance with Serianni et al. [Citation11].
RESULTS
No adverse effects of DDFPe infusion were observed in the recordings of HR, RF, and blood pressures during any of the experiments that lasted up to 12 h. Results regarding PaO2, PaCO2, and pHa are depicted in , PmO2 and PmCO2 in , CVP and RVP in , and PAP in .
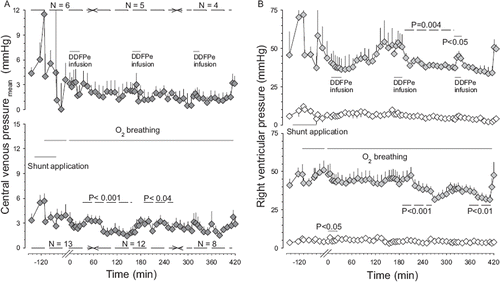
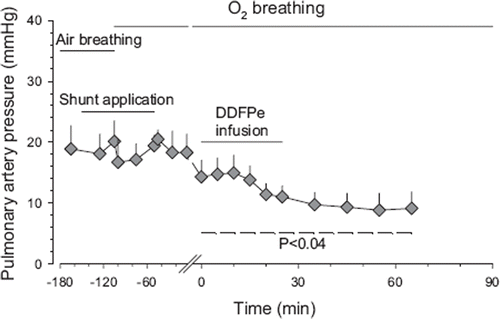
Figure 4. Average pulmonary artery pressure versus time in Hsat-Group and Lsat-Group pigs combined during air breathing (control) before and after shunt application and subsequent oxygen breathing and 30 min after the first infusion of DDFPe. All values are given as means±SEM (for further explanations see legend).
Control Phase
During the pre-shunt air-breathing control phase, the arterial oxygen tension (PaO2) was 71.9±2.7 mmHg, which kept the O2 saturation (SaO2) at 90±1%, adjusted to pig blood characteristics [Citation11]. At the same time, the arterial carbon dioxide tension (PaCO2) was 43.2±1.1 mmHg and the pH was 7.448±0.008. The muscle oxygen tension (PmO2) was 39±3 mmHg and the muscle carbon dioxide tension (PmCO2) was 61±4 mmHg. The other parameters were: mAP 115±6 mmHg, CVP 3.6±0.6 mmHg, peak RVP 42±4 mmHg, and mPAP 18±4 mmHg; HR was 153±8 beats/min, and RF 32±3 breaths/min. The somewhat low PaO2 during air breathing before shunt application was, in all likelihood, due to deterioration of the pulmonary ventilation/perfusion distribution brought about by the abnormal supine position of the spontaneously breathing, anesthetized animals.
Effect of Shunt Application
Administration of the beads, during the air breathing in the pretreatment control phase, typically caused the PaO2 to fall by 54% (P<0.001) from the earlier mentioned 71.9±2.7 to 31.8±1.4 mmHg, which corresponded to a reduction in arterial O2 saturation by 49% (P<0.001) to 47±3%. The PaCO2 increased 37% (P<0.001) to 59.0±2.9 mmHg, the pH decreased (P<0.001) to 7.326±0.021 and the PmO2 fell 45% (P<0.001) to 21±3 mmHg and PmCO2 increased 6% (P<0.04) to 65±4 mmHg. During shunt application and air breathing, nine of the pigs with the most marked increases in PaCO2 stopped breathing and artificial ventilation had to be administered. Of these pigs, six returned to spontaneous breathing 2–6 min after 100% O2 was administered, while three had to be ventilated ∼5min into the DDFP treatment period before they resumed spontaneous breathing.
As for blood pressures during shunt application while still breathing air, the mAP had increased 21% (P<0.002) to 137±6 mmHg (probably a hypoxic effect), HR was unchanged at 158±7 beats/min, mean CVP had doubled (P=0.001) to 7.4±1.5 mmHg, while peak RVP at 54±8 mmHg, mPAP at 18±2 mmHg and RF at 32±4 breaths/min underwent no significant changes.
Upon bead administration combined with O2 breathing, the PaO2 increased (P<0.001) to 75.1±8.0 mmHg giving a saturation of 85±4%, i.e. essentially equaling the control level during air breathing before the shunt was applied. At this time PaCO2 had increased by 61% (P<0.001) to 69.6±5.9 mmHg and tissue PmCO2 by 28% (P<0.001) to 78±6 mmHg, compared to control levels. The pH fell further (P<0.001) to 7.271±0.030. These indicators of hypercapnia were, in all likelihood, caused by anesthesia-induced hypoventilation and/or deterioration in the ventilation/perfusion distribution due to partial obstruction of some airways.
After shunt application was completed and the pigs had been breathing oxygen for approximately 30 min the HR was unchanged at 175±6 beats/min and the blood pressure readings were again at control levels with the mAP 120±6 mmHg, mean CVP 3.8±1.1 mmHg, peak RVP 49±5 mmHg, mPAP 18±3 mmHg and the RF at 38±6 breaths/min.
Preliminary inspection of the data when all experiments were completed suggested that animals differed in response to the bead and oxygen administration. Therefore it was arbitrarily decided to divide the 19 animals into two groups, based on the shunt effect on arterial oxygen saturation, one with a pre-treatment SaO2> 90% (high saturation group or Hsat-Group, n=6) and one with pre-treatment SaO2 <90% (low saturation group or Lsat-Group, n=13) ().
Table 1. Arterial and venous oxygen tensions and saturations in pigs during oxygen breathing after shunt application, before and during DDFPe treatment
Except for mPAP values (), which were calculated as the average of 6 animals (3 from each group), the results are reported for each group separately.
Effect of DDFPe Infusions
Within five min from the beginning of the first DDFPe infusion (∼0.5 ml given), the PaO2 started to increase in both groups. The previously mentioned three animals with high PaCO2 levels and respiratory arrest during O2 administration (only) began to breathe spontaneously again after 5–10 min of DDFPe infusion and artificial ventilation. In these three animals, the PaCO2 and PmCO2 fell during the infusion.
Absolute values for changes in arterial and venous blood oxygenation are offered in and changes in various physiological parameters related to time are depicted in and (regarding , see Discussion).
Figure 5. This figure is based on theoretical calculations (see above) illustrating the effect, during oxygen breathing, of surface tension on the dose-response curve of DDFP emulsions in the treatment of hypoxia resulting from a 60% right to left shunt.
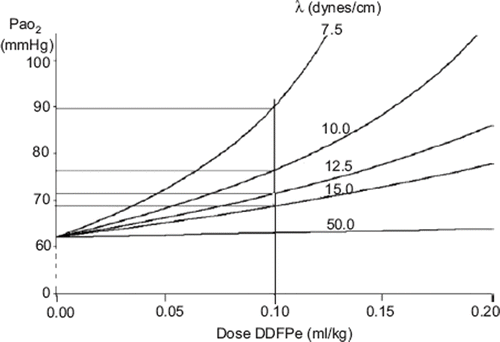
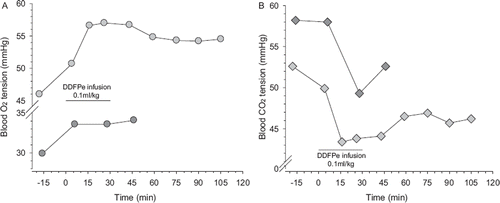
Figure 6. Panel A: arterial oxygen tension (PaO2), light grey circles and, venous oxygen tensions (PvO2), dark grey circles versus time in air-breathing pig with nosocomial pneumonia before, during and after infusion of 0.1 ml/kg (body weight) of DDFPe. Panel B: arterial carbon dioxide (PaCO2), light grey diamonds, and venous carbon dioxide (PvCO2) tensions, dark grey diamonds, in the same pig.
Hsat-Group
During the first DDFPe infusion PaO2 increased by 45% and SaO2 by 39% (), compared to the last readings before treatment started, while PaCO2 and pH showed no significant changes. The PvO2 resulting from the first DDFPe infusion rose 12% and the resulting SvO2 by 11% (). PmO2 increased up to 56% (P<0.001) and PmCO2 was unchanged as were HR, CVP, mAP and RF. RVP displayed a falling trend (∼10%); mPAP, however, fell by 24% (P=0.036).
During the second DDFPe infusion only PaO2 and PmO2 increased, 44% (P<0.01) and 25% (P<0.03), respectively. No other parameters showed significant changes.
During the third DDFPe infusion increases were again noted in PaO2, this time by 88% (P=0.02). With the exception of RVP, which had a temporary increase of 18% during infusion (P<0.05), all other parameters were unchanged.
Lsat-Group
During the first DDFPe infusion PaO2 increased by 39% (P<0.001), PaCO2 fell by 86% (P=0.02) at the end of the infusion and pH increased from 7.266±0.046 to 7.320±0.038 (P=0.002). The changes in venous oxygenation included an increase in PvO2 by 20 % and SvO2 by 22% (cf. ). PmO2 increased by 35% (P<0.001); PmCO2 was down 11% at the end of the infusion (P=0.02); mAP fell on average by 9% (P=0.02) during the infusion while HR, CVP, RVP as well as RF remained unchanged.
During the second DDFPe infusion PaO2 increased by 26% (P<0.001) and PmO2 by 22% (P<0.003). No other parameters showed significant changes.
During the third DDFPe infusion both PaO2 and PmO2 increased by 28% (P<0.001) and 19% (P=0.002), respectively. The other parameters remained unchanged.
DDFPe Washout
Hsat-Group
After the first infusion average PaO2 exhibited an increase (∼40%, n.s.). The treatment effect shown by PmO2 declined during the next hour from 38%, 20 min post infusion, to 11% (P<0.02). An elevated O2 tension was observed in some pigs also during the second hour, although the average was no longer significantly increased. Of the pigs in the group that were given two (n=5) or three (n=4) treatments all but one pig showed decreased levels of PaO2 by the end of the observation periods compared to values before any infusions were given. During the first hour after completed treatment the PaCO2 was lower by up to 5%, after which it started to increase above pre-treatment level (P<0.001). In the muscle the CO2 tension was unchanged until the last third of the observation period (preceding the next infusion), when it gradually rose (24%, P<0.001). The arterial pH became more alkaline compared to pretreatment values during this time period (P=0.011). Reduction in heart rate up to 13% was recorded in the middle of the time period (P<0.02), but no other parameters were significantly changed after the first infusion.
After the second infusion the elevation in PaO2 (11–78%) persisted for the next 2 hrs (P=0.007), but PaCO2 and pH remained unchanged compared to pretreatment values. Remarkably, PmO2 did not increase in this period and PmCO2 was not significantly changed either. RVP was reduced up to 19% during the two-hour observation period (P<0.004), while mAP, CVP, and RF remained unchanged.
After the third infusion the PaO2 remained elevated by 45–100% for the next 2 hrs (P<0.05) while PaCO2 stayed the same. The pH on the other hand was increased (P<0.04) compared to pretreatment values. However, PmO2 did not reflect the elevated O2 levels. The PmCO2 was not changed by the treatment, neither were mAP, CVP, RVP or HR and RF.
Lsat-Group
After the first infusion significant increases (17–45%) in PaO2 (P<0.02) and PmO2 (18–41%) (P<0.04) persisted for the next two hours. During the first 80 min after completed treatment the PaCO2 remained reduced by 11–16% (P<0.001), after which it returned toward pre-treatment levels. In the muscle, CO2 was reduced up to 16% (P<0.02) during the period between the first and second treatments while pH was elevated (P<0.001). The CVP was reduced by ∼50% (P<0.001) during the last hour of the observation period, while there were no changes in mAP, RVP, HR, or RF.
With the second infusion similar changes occurred in PaO2, PaCO2, and pH as after the first infusion: PaO2 increased up to 50% (P<0.004), PaCO2 showed a slow increase in tension (P<0.001) over time, while pH remained at the same level. The average PmO2 showed an increasing trend but not all pigs maintained the increase in O2 tension that they had during the treatment infusion. The PmCO2 remained unchanged. CVP remained lowered for up to 80 min after the end of the treatment infusion and RVP kept falling (up to 30% reduction, P<0.001) 90 min into the observation period before the pressure started to increase again. No other parameters showed changes.
After the third infusion the PaO2 stayed elevated by 23–44% for the next 2 hrs (P<0.023) while PaCO2 and pH were unchanged compared to pretreatment values. PmO2 did not, however, reflect the elevated O2 levels. The PmCO2 was unaffected by the treatment. All other parameters were unchanged, except for RVP, which showed reductions (P<0.01) in the last half of the observation period before the animals were returned to air breathing.
Return to Air Breathing
When the treatment-improved oxygenation had subsided and the animals were returned to air breathing, five animals (all from Lsat-Group) stopped breathing within 5 min. Before 10 min had passed, 3 more pigs became apneic and after 15 min only seven out of 19 pigs were still breathing spontaneously. All animals were euthanized shortly after they ceased breathing, according to protocol. The main responses to air breathing, compared to the oxygen breathing previously, were a dramatic drop in PaO2 (P<0.001) and PmO2 (P<0.001), as well as drop in PaCO2 (P=0.035) due to hyperventilation, but no change in PmCO2. HR increased, and increases in all blood pressures were recorded. Compared to values during bead application while the pigs were still breathing air, there was no difference in PaO2 or PaCO2; pHa, however, had increased (P<0.03). PmO2 and PmCO2 were largely unchanged, but mAP and CVP were reduced 24% (P<0.03) and ∼50% (P=0.002), respectively. RVP, HR, and RF (in the animals still breathing spontaneously) were mostly at the same levels as during air breathing before any treatment was administered.
On autopsy, a diffusely distributed patchy pattern or larger areas of non-aerated lung tissue (i.e. atelectasis) were observed both under the visceral pleura and on the cut surfaces of the lungs.
DISCUSSION
The main findings in the present study strongly indicate that DDFP-derived microbubbles generated intravascularly were able to significantly alleviate the arterial hypoxia resulting from the experimental pulmonary right-to-left shunt in pigs. The present study uses increases in PaO2 and PvO2 or the derived SaO2 and SvO2 as indicators of the ability of DDFPe treatment to relieve shunt-induced arterial hypoxia. To appreciate this approach it should be recalled that, relative to fully oxygenated pulmonary capillary blood, the arterial oxygen concentration is depressed by the lower oxygen level in the shunted venous blood. The introduction of DDFP bubbles, which take up oxygen in the lung capillaries, boosts the oxygen content in the arterial blood. Hence, the unloading of hemoglobin-bound oxygen in the tissues will be diminished, which allows for an increased oxygen level in the venous blood, even if the oxygen extraction in the tissues might be somewhat increased in response to improved oxygen availability (cf. ). When this venous blood is then mixed with pulmonary capillary blood, higher PaO2, and SaO2 levels are achieved than in the absence of bubbles.
In retrospect, the animals in Hsat-Group received bead doses that were less than ideal, resulting in PaO2 levels at around 110 mmHg during oxygen breathing after bead administration. This suggests that they had a relatively marked component of ventilation/perfusion ![]() mismatch. However, the fact that the PaO2 was far lower than the ∼ 500 mmHg to be expected if the situation were a pure mismatch indicates that these animals had a substantial shunt component in their lung function.
mismatch. However, the fact that the PaO2 was far lower than the ∼ 500 mmHg to be expected if the situation were a pure mismatch indicates that these animals had a substantial shunt component in their lung function.
It is unlikely that the PaO2 increase, in response to treatment, could have been due to the shunt becoming smaller in the course of the experiment since, after return to air breathing at the end of experiments, the final PaO2 was at the same level as during air breathing following the shunt application. An alternative mechanism would be an increase in cardiac output reducing the oxygen extraction from an increased flow of arterial blood through the systemic circulation. While debatable, an argument against the latter explanation is that the cardiac output of intact, anesthetized, and oxygen breathing pigs has been reported not to change significantly after DDFPe treatment [Citation12]. The first above mentioned explanation of the DDFPe effect is in agreement with our earlier in vitro demonstration that DDFPe derived microbubbles can, by diffusion, exchange oxygen with a surrounding aqueous medium [Citation1]. Additional support for this mechanism is offered by the following theoretical considerations: On the proviso that the fully developed bubbles are small enough to pass through the capillaries, the effectiveness of a dose of DDFPe to increase the transport of oxygen to the tissues depends on how much oxygen is taken up in the lungs by the initially pure DDFP bubbles as dissolved O2, CO2, and N2 in the blood diffuses into them. The final size is determined by the balance between the sum of the partial pressures of those gases and the outside pressure that is tending to compress the bubbles. The latter pressure is equal to the sum of the barometric pressure, blood pressure, and the pressure resulting from surface tension.
Applying this concept to bubbles in the pulmonary and peripheral capillaries, where the blood pressure is minimal, yields the following:
where R, Pb, γ, and 47 represent bubble radius, barometric pressure, surface tension, and water vapor pressure, respectively, while c and v represent the beginning and end of the pulmonary cappillary, and a represents the arterial blood.
If we assume that DDFP diffuses extremely slowly out of the bubble (due to its very low water solubility), while pulmonary end capillary and alveolar O2, CO2, and N2 pressures are in equilibrium then:
Combining equations (1a) and (2), ![]() Applying the ideal gas law:
Applying the ideal gas law:
where 1.66 is the density and 288 is the molecular weight of DDFP, and Vpart and Rpart represent the volume and radius of the DDFP particles in the DDFP emulsion.
Equation (4) implies that the size of the bubbles leaving the pulmonary capillaries depends on the surface tension. This is illustrated in Figure 5, which plots the effect of surface tension on the dose-response curve of DDFP emulsions in the treatment of shunt-caused hypoxia that is similar in degree to that observed in the Lsat-Group animals. A dose of 0.03 g/L approximates the dose in the experiments described above. The curves shown correspond to assigned surface tensions of 7.5, 10.0, 12.5, 15.0, and 50 (plasma) dynes/cm. Note that to get any improvement in PaO2 in the presence of DDFP bubbles, the surface tension must be substantially lower than that of plasma. Such a lowering of surface tension is likely to be imparted by the surfactant which is part of the composition of the preparation [Citation7].
As for the performance of the DDFPe in the present preparation, two possibilities seem probable. One is the earlier mentioned, which is dependent on the increased oxygenation of pulmonary capillary blood due to the microbubbles and the resulting increase in venous and subsequently arterial PO2. Alternatively, the treatment might somehow serve to further accentuate the natural tendency to reduced blood flow through inadequately ventilated or, in this case, atelectatic lung areas. This could perhaps be brought about by microbubbles blocking partially constricted (due to local hypoxia) vessels in which the diameter has become less than the bubble diameters. However, supporting the first mentioned mechanism are our earlier cited observations (e.g. 1) of favorable effects of DDFPe treatment of conditions in which hypoxia is likely to be due to other mechanisms than shunts or ![]() inequalities. In the present study, already after ∼1 min of DDFPe infusions, the PaO2 started to increase. After 5 min of the first infusion, PaO2 had increased 41% in Hsat-Group and leveled out at 46%. In Lsat-Group, on the other hand, the increase after 5 min was only 23% but it eventually reached 51%. These peaks in PaO2 were seen 80–90 min after the infusion was terminated in both groups (45% increase compared to pretreatment levels in both groups) (). Approximately 2 hrs later, the PaO2 approached the control values recorded before the infusion. During the first infusion, the muscle O2 tension followed approximately the same pattern () as the one in arterial blood. After 5 min the increases were 20 and 31%, respectively, while the maximum increases were 56 and 43%. In the muscle tissue, the increase vanished in Hsat-Group after 80 to 90 min, while in Lsat-Group the effect persisted, although somewhat reduced, until the next infusion. These observations closely resemble the effects on muscle PO2 of comparable doses of DDFPe given to intact rats during oxygen breathing, and even the duration of the effect was similar in the rats, i.e. about 2.5 hr [Citation12]. Further support for the preparation’s ability to enhance circulatory oxygen levels has been obtained in the earlier-mentioned intact pigs that were administered oxygen during studies of DDFPe effects on tissue nitrogen elimination. In those animals, given 0.08 ml/kg of DDFPe, the mixed venous PO2 increased by about 50% [Citation1].
inequalities. In the present study, already after ∼1 min of DDFPe infusions, the PaO2 started to increase. After 5 min of the first infusion, PaO2 had increased 41% in Hsat-Group and leveled out at 46%. In Lsat-Group, on the other hand, the increase after 5 min was only 23% but it eventually reached 51%. These peaks in PaO2 were seen 80–90 min after the infusion was terminated in both groups (45% increase compared to pretreatment levels in both groups) (). Approximately 2 hrs later, the PaO2 approached the control values recorded before the infusion. During the first infusion, the muscle O2 tension followed approximately the same pattern () as the one in arterial blood. After 5 min the increases were 20 and 31%, respectively, while the maximum increases were 56 and 43%. In the muscle tissue, the increase vanished in Hsat-Group after 80 to 90 min, while in Lsat-Group the effect persisted, although somewhat reduced, until the next infusion. These observations closely resemble the effects on muscle PO2 of comparable doses of DDFPe given to intact rats during oxygen breathing, and even the duration of the effect was similar in the rats, i.e. about 2.5 hr [Citation12]. Further support for the preparation’s ability to enhance circulatory oxygen levels has been obtained in the earlier-mentioned intact pigs that were administered oxygen during studies of DDFPe effects on tissue nitrogen elimination. In those animals, given 0.08 ml/kg of DDFPe, the mixed venous PO2 increased by about 50% [Citation1].
No signs of pulmonary embolization by DDFP-derived bubbles were observed in the present experiments. On the contrary, as seen in , the PAP actually fell in response to DDFPe infusion (P<0.04), conceivably due to vascular dilatation induced by higher PO2 in vessels supplying poorly ventilated areas.
In the course of the three-tiered treatment sequence, a step-wise increasing effect on PaO2-levels occurred. This phenomenon is particularly evident in , Lsat-Group, which shows significant increases between infusions. This might reflect a gradual build-up of DDFP in tissues, which thus may have served as a DDFP-store, slowing bubble shrinkage and disappearance. Although DDFP elimination ultimately occurs via expired air, diffusional exchange of DDFP between bubbles and tissues is to be expected and has been indirectly confirmed by serial gas-chromatographic analyses of DDFP in various tissue samples from pigs (unpublished observations). While DDFP concentrations fall with time, DDFP-traces have been recorded after up to 6 hrs following the last infusion.
That the artificial shunt model is likely to be clinically relevant and the DDFPe useful for treating right-to-left shunts in humans gains support from serendipitous observations in an air-breathing pig that was intended to be part of this study but which, during the early phase of surgical preparation, was disqualified because it had a very low PaO2 (46 mmHg). On the assumption that the animal suffered from a condition causing shunting, DDFPe treatment was administered to the continuously air breathing animal according to the same schedule employed in the healthy animals with experimental shunts. The resulting 10 mmHg increase in PaO2 and approximately equivalent decrease in PaCO2 are illustrated in . These improvements lasted throughout an approximately 2-hr observation period, which was terminated by euthanasia. Based on the findings in the immediately performed autopsy, the hypoxia and hypercapnia before treatment were consistent with the presence of purulent pneumonia, engaging an estimated 75% of the lungs.
The DDFPe, originally developed by Sonus Pharmaceuticals, Inc., has undergone successful human testing as an ultrasound contrast medium. The possibility of safely using this type of emulsion, for therapeutical purposes, in humans gains some credence from previous testing showing the preparation lacked significant adverse effects when administered in bolus doses of up to 0.35 ml/kg in 743 patients/volunteers [Citation13] and in subsequent studies using doses of 0.05 and 0.12 ml/kg in an additional 1200 subjects [Citation14]. The dose used in the present study (0.1 ml/kg) is comparable to the highest one used in the aforementioned testing and it is reasonable to expect that the slow infusion will add further safety compared to the bolus injentions employed with contrast media.
CONCLUSIONS
If the additional safety testing required for the approval of this type of preparation in human medicine is successful, a dodecafluoropentane emulsion may become a valuable adjuvant for treating arterial hypoxia of various etiologies.
Declaration of interest: Authors I. Tyssebotn and C. Lundgren are two of four inventors on US patents Nos. 6.127,428 and 5,869,538 pertaining to the use of micro-bubbles for enhancing circulatory gas transport in the body. These patents are owned by the Research Foundation (RF) of the State of N.Y. which has licensed the technology to a pharmaceutical company for commercialization. The inventors will receive part of any income received by the RF from the licensee.
REFERENCES
- Lundgren, C.E.G., Bergoe, G., Tyssebotn, I.M. (2006). Intravascular fluorocarbon-stabilized microbubbles protect against fatal anemia in rats. Artificial Cells, Blood Substitutes, and Biotechnology, an International Journal 34:473–486.
- Koch, J.C., Oprysko, P.R., Shuman, A.L., Jenkins, W.T., Brandt, G., Evans, S.M. (2002). Radiosensitization of hypoxic tumor cells by dodecafluoropentane: a gas-phase perfluorochemical emulsion. Cancer Research 62:3626–3629.
- Burkard, M.E., Van Liew, H.D. (1994). Oxygen transport to tissue by persistent bubbles: Theory and simulations. J Appl Physiol 77:2874–2878.
- Van Liew, H.D., Burkard, M.E. (1996). Relationship of oxygen content to PO2 for stabilized bubbles in the circulation: theory. J Appl Physiol 81:500–508.
- Correas, J.M., Quay, S.D. (1996). EchoGen™ emulsion: A new ultrasound contrast agent based on phase shift colloids. Clinical Radiology 51(Suppl. 1):11–14.
- Lopez-Ben, R., Robin, M.L., Weber, T.M., Smith, J.K., Needleman, L., Berland, L.L. (1999). Doppler sonographic enhancement of hepatic hemangiomas and hepatocellular carcinomas after perflenapent emulsion: preliminary study. J Ultrasound Med 18:109–116.
- Quay, S. C. (1999) Composition comprising a fluorine containing surfactant and perfluoropentane for ultrasound. US patent No. 5,876,696.
- Olszewski, R., Marciniak, W., Nowicki, A., Etienne, J., Karlowicz, P., Adamus, J. (1998). The improvement of endocardiographic assessment of left ventricle by the use of permaflent and harmonic imaging. Polski Merkuriusz Lekarski 5:132–34.
- Walker, K.W., Pantely, G.A., Sahn, D.J. (1997). Ultrasound-mediated destruction of contrast agents. Effect of ultrasound intensity, exposure and frequency. Investigative Radiology 32:728–34.
- Eyal, F.G., Hachey, W.E., Curtet-Eval, N.L., Kellum, F.E., Alpan, G. (1996). Effect of modulators of hypoxic pulmonary vasoconstriction on the response to inhaled nitric oxide in a neonatal model of severe pulmonary atelectasis. Semin Perinatol 20:186–93.
- Serianni, R., Barash, J., Bentley, T., Sharma, Fontana, J.L., Via, D., Duhm, J., Bunger, R., Mongan, P.D. (2003). Porcine-specific hemoglobin saturation measurements. J Appl Physiol 94:561–566.
- Lundgren, C., Bergoe, G., Olszowka, A., Tyssebotn, I. (2005). Tissue nitrogen elimination in oxygen-breathing pigs is enhanced by fluorocarbon-derived intravascular micro-bubbles. Undersea and Hyperbaric Medicine 32:215–226.
- Quay, S.C., Eisenfeld, A.J. (1997). Safety assessment of the use of perflenapent emulsion for contrast enhancement of echocardiography and diagnostic radiology ultrasound studies. Clinical Cardiology 20(Suppl. 1): I19–26.
- Personal communication. (2005) From the then Marketing and Business Manager, Mr. Mark Low, at Sonus Pharmaceuticals, Inc.