Abstract:
A novel encapsulated oxidative biocatalyst comprising glucose oxidase (GOD) coencapsulated with oxygen carriers within polyelectrolyte complex capsules was developed for the production of D-gluconic acid and δ-gluconolactone. The capsules containing immobilized GOD were produced by polyelectrolyte complexation with sodium alginate (SA) and cellulose sulfate (CS) as polyanions, poly(methylene-co-guanidine) (PMCG) as the polycation, CaCl2 as the gelling agent and NaCl as the antigelling agent (GOD-SA-CS/PMCG capsules). Poly(dimethylsiloxane) (PDMS) and an emulsion of n-dodecane (DOD) or perfluorodecaline (PFD) with PDMS were used as the oxygen carriers and MnO2 was used as a hydrogen peroxide decomposition catalyst. Water-soluble PDMS was found to act as both an oxygen carrier and an emulsifier of water-insoluble DOD and PFD. Stable microcapsules could be produced with concentrations of up to 4% (w/w) of PDMS, 10% (w/w) of DOD and PFD, and 25% (w/w) of MnO2 in the polyanion solution of SA and CS. Roughly a two-fold increase in the GOD activity from 21.0±1.1 to 38.4±2.0 U·g−1 and product space-time yields (STY) from 44.3±2.0 to 83.4±3.4 g·H·day−1 could be achieved utilizing coencapsulated oxygen carriers compared to GOD encapsulated in the absence of oxygen carriers. This enhanced production does not significantly depend on the selected oxygen carrier under the conditions used in this study.
INTRODUCTION
D-gluconic acid is considered to be one of the most important organic chemicals produced from sugar [Citation1]. Efficient enzymatic production of D-gluconic acid via biooxidation from D-glucose is catalyzed by the glucose oxidase-catalase (GOD-CAT) system and requires permanent saturation by oxygen as a cosubstrate ().
Figure 1. Chemical equation of glucose oxidation catalysed by glucose oxidase (GOD) and catalase (CAT), compiled according to [Citation2].
![Figure 1. Chemical equation of glucose oxidation catalysed by glucose oxidase (GOD) and catalase (CAT), compiled according to [Citation2].](/cms/asset/83526122-9697-4e9d-9c8d-3ea97939cb02/ianb19_a_463983_f0001_b.gif)
Two persistent challenges of this biooxidation process include low oxygen solubility in aqueous solutions [Citation2] and GOD inactivation by hydrogen peroxide produced in situ [Citation3]. Both effects result in reduced space-time yields (STY) of the D-gluconic acid product. Major efforts have been made to improve the oxygen mass transfer by increasing the oxygen partial pressure [Citation4], optimizing the oxygen bubble size and turbulence of the gas flow [Citation5], increasing the oxygen supply by hydrogen peroxide decomposition using catalase [Citation3] or MnO2 [Citation2,Citation6], and using water-soluble oxygen carriers, as well [Citation7]. Utilization of bubble-free aeration systems for oxygen saturation is also possible; however, it is restricted to the oxygen mass transfer resistance on the boundary layers on either side of the aeration membranes [Citation8]. Nevertheless, it is evident that oxygen saturation using pure oxygen and the continuous measurement of oxygen concentration is missing in the majority of biooxidation studies that utilize immobilized GOD. The lack of these two actions may hinder progress in the field of GOD encapsulation, since the oxygen solubility in aqueous solutions is a rate-limiting factor in biooxidation reactions catalyzed by both free and immobilized GOD [Citation2].
Recently, it was shown that the encapsulation of GOD in the polyelectrolyte complex capsules composed of sodium alginate (SA), cellulose sulfate (CS), poly(methylene-co-guanidine) (PMCG), CaCl 2 and NaCl (SA-CS/PMCG capsules) [Citation2] is made possible when the pore size of the capsular membrane is lower than the size of the encapsulated enzyme. This feature is a prerequisite for a successful enzyme encapsulation without additional preimmobilization steps, which may decrease the enzyme activity [Citation9]. SA-CS/PMCG capsules represent a multi-purpose immobilization matrix. These capsules were developed for encapsulation of islets of Langerhans [Citation10], but can be successfully used for encapsulation of other whole-cell biocatalysts, as well [Citation11,Citation12].
The focus of this study was a preparation of SA-CS/PMCG capsules with a GOD biocatalyst co-immobilized with oxygen carriers, which is an extension of our previous study with GOD immobilized within SA-CS/PMCG capsules [Citation2]. The main aim was to increase the oxygen level within the capsules to improve the space-time yield of produced D-gluconic acid and δ-gluconolactone. Coimmobilization of oxygen carriers together with biocatalysts is not a novel concept, which was previously used for encapsulation of oxygen carriers together with whole-cells in alginate beads [Citation13,Citation14]. The novelty of this work is encapsulation of GOD together with water-soluble poly(dimethylsiloxane) (PDMS), acting both as an oxygen carrier and an emulsifier of other oxygen carriers n -dodecane (DOD) or perfluorodecaline (PFD), within SA-CS/PMCG microcapsules. In addition, the effect of MnO2 (a hydrogen peroxide decomposition catalyst) coencapsulated with GOD on the enzyme activity was also examined.
MATERIALS AND METHODS
Glucose oxidase (GOD, EC 1.1.3.4, preparation from Aspergillus niger) with a specific activity of 199 U·mg−1 and a GOD/CAT ratio of 18,440 was obtained from Biozyme Laboratories Ltd. (Gwent, UK). High viscosity sodium alginate (SA) was kindly donated from ISP Alginates (Girvan, Ayrshire, UK). The limiting viscosity number for SA (8.4 dl·g−1 in 0.1 M aqueous NaCl solution at 25°C) and molecular weight (420 kDa) were determined as reported previously [Citation11]. Cellulose sulfate (CS; sodium salt) was from Acros Organics (New Jersey, USA); the average degree of substitution of 1.7 was determined for CS as reported previously [Citation11]. Poly(methylene- co-guanidine hydrochloride) (PMCG) (Scientific Polymer Products Inc., Ontario, NY, USA) supplied as a 35% (w/v) aqueous solution was lyophilized prior to use. The molecular weight of PMCG (4.5 kDa) was determined as reported previously [Citation11] . Compressed oxygen (purity 99.5%) was purchased from SIAD (Bratislava, Slovakia). Perfluorodecaline purum (PFD) was from Fluka (Buchs, Switzerland), n-dodecane (DOD) was supplied from Merck (Hohenbrunn, Germany). Poly(dimethylsiloxane) (PDMS) copolymer was a gift from Dr. E. S. Dey (Lund University, Lund, Sweden), which is a product of Dow Corning (USA, Cat. No. Q2-5247). The PDMS copolymer with a molecular mass of 27.9 kDa and a kinematic viscosity of 2.3.103 m2. s1 contained 18% of dimethyl siloxane, 35% of ethylene oxide and 46% of propylene oxide [Citation7]. Pluronic F-68 was from Sigma-Aldrich (St. Louis, MO, USA).
Encapsulation of GOD in SA-CS/PMCG Capsules
GOD encapsulation was performed using a custom-made coaxial air-stripping extrusion device fitted with a multiloop reactor [Citation15], as reported previously [Citation2]. The polyanion (PA) solution was prepared from 0.9% (w/v) SA and 0.9% (w/v) CS in 0.9% (w/v) NaCl at pH 7.0. GOD was dissolved in the PA solution at a concentration of 1 mg.ml1. Subsequently, 20 ml of the PA solution with GOD was centrifuged at 4,000 min1 and 23°C for 1.5 h to remove insoluble impurities. Drops of the PA solution containing GOD were extruded at an air pressure of 0.4 bar, a flow rate of stripping air in a coaxial nozzle of 1.5 1·min1 , and a PA flow rate of about 0.5 ml·min1 into the stream of a polycation (PC) solution that was continuously flowing in the 5-loop reactor. The PC solution consisting of 1.8% (w/v) PMCG, 1.0% (w/v) CaCl 2 and 0.9% (w/v) NaCl was delivered from the 5 1 Container into the multiloop reactor at an air pressure of 0.1 bar and a flow rate of 50 ml·min1 providing the reaction time for capsule formation of 70 s. The reaction was quenched by collecting of GOD-SA-CS/PMCG capsules at the exit of the reactor in 150 ml of 0.9% (w/v) NaCl in 1 min intervals. The capsules were then treated for 5 min with an excess of 50 mM citrate solution in 0.9% NaCl, followed by washing in 0.9% (w/v) NaCl. The second polyanion layer of the capsular membrane was prepared by subsequent 10 min treatment of the capsules with 0.1% (w/v) solution of CS in 0.9% (w/v) NaCl followed by washing of the capsules with 0.9% (w/v) NaCl. The final GOD-SA-CS/PMCG capsules were stored at 4°C. Capsule imaging and determination of an average size and membrane thickness for batches of 20 capsules were performed using an optical microscope LM6-3 or a stereomicroscope STM 723 (both from Kvant, Bratislava, Slovakia) equipped with a digital camera Olympus C-4000 ZOOM (Tokyo, Japan) [Citation11]. The camera was interfaced to a PC operating with Impor 5.0 Professional Image Analysis software (Kvant, Bratislava, Slovakia). Values for two perpendicular diameters and four membrane thicknesses at positions 90° apart were taken for each capsule [Citation11].
Coencapsulation of GOD and Oxygen Carriers or MnO2 in SA-CS/PMCG Capsules
Preparation of the capsules with GOD coencapsulated with either oxygen carriers or MnO2 was performed using the same encapsulation protocol as described above. Centrifugation of the PA solutions was avoided to prevent separation of oxygen carriers or sedimentation of MnO2 within the PA solution. The PA solutions contained GOD and the following amount of chemical agents: a) 4% (w/w) PDMS, b) 4% (w/w) PDMS and 10% (w/w) PFD, c) 4% (w/w) PDMS and 10% (w/w) DOD, d) 5% (w/w) MnO2, e) 10% (w/w) MnO2, f) 25% (w/w) MnO2. The PA solutions with oxygen carriers or MnO2 were thoroughly mixed prior to encapsulation.
Biooxidations
D-glucose solutions (7.2 ml) of different concentrations in sodium phosphate buffer (0.05 M, pH 6) supplemented with oxygen (0.625 mM) were added to 800 mg of wet GOD-SA-CS/PMCG capsules in a model bioreactor (reactor volume of 24 ml). The double-jacketed reactor was thermostated (30°C) and equipped with a cylindrical oxygen sparger for oxygenation and mixing, to keep capsules in fluidized bed-like conditions. Buffer aliquots (60 µl) were periodically withdrawn and analyzed by the HPLC method as described below. The biooxidation reactions were carried out for 5 hours.
Glucose Monitoring by a Flow Calorimetry
A flow microcalorimeter (FC) (3300 Thermal Assay Probe, Advanced Biosensor Technology, AB, Lund, Sweden) was used for monitoring of glucose consumption as described previously [Citation16,Citation17]. The main part of the system is a thermostatic cell containing the column filled with the immobilized enzyme. The column is operated as a small packed bed reactor. The temperature difference between the column input and Output, ΔT, is measured by a thermistor. The column (7 mm i.d. × 20 mm) was packed with wet capsules containing GOD (500 mg of wet weight). Initially, thermostated (30°C) phosphate buffer (0.05 M, pH 6.0) saturated with oxygen (0.3 l min1) was passed through the system at a flow rate 1 ml min1 until the thermal equilibration temperature (30°C) was reached. After thermal equilibration, 9 ml of phosphate buffer was recirculated (by switching of the valve from the waste to the recirculation loop returning the reaction mixture to a stirred reservoir) [Citation16] through the stirred microreactor that was filled with 680 mg of tested GOD-SA-CS/PMCG capsules with or without oxygen carriers coencapsulated. The mobile phase was continuously saturated with oxygen at 30°C by oxygen bubbling. After steady ΔT was obtained, the reaction, which took 5 h was initiated by the addition of 1 ml glucose (1 M). The temperature change due to the oxidation reaction was calculated from the height of the thermogram using the Labtech software [Citation16]. The concentrations of glucose were calculated from the thermometric signal according to the autocalibration method, as described previously [Citation16] with the follow-ing Parameters: vm=57.7±1.1 mK, Km=3.0±0.18 mM, Ki=2.8±3.3·10−4 mM. Simultaneously, the glucose concentration in samples withdrawn from the stirred reservoir was analyzed by HPLC.
Determination of GOD Activity in an Immobilized Form
The activity of immobilized GOD was determined spec-trophotometrically by the method based on horseradish peroxidase-coupled oxidation of o-dianisidine as previously described [Citation2] assay suspension consisted of 8.7 ml of 0.1 M potassium phosphate buffer, pH 6, containing 0.725 mg of o-dianisidine, 0.33 ml of a horseradish peroxidase solution (0.2 mg·mk1), 5 mg of wet immobilized GOD was saturated with oxygen for 10 min by oxygen bubbling. The enzyme reaction was initiated by the addition of 1 ml of 18% (w/v) D-glucose at 30°C. One unit of GOD activity corresponds to the oxidation of 1 μmol of o-dianisidine (measured as the change in the absorbance at 460 nm) in 1 min under conditions used.
High-performance Liquid Chromatography (HPLC)
HPLC was used to determine the amount of D-gluconic acid and δ-gluconolactone produced and D-glucose consumed, as well. The HPLC system Shimadzu (Shimadzu, Wien, Austria) equipped with a high pressure pump LC-10 AD, a membrane degasser GT-104, an injector Rheodyne 7725i with a 20 μl loop, an UV-VIS detector SPD-10AV and RI detector RID-6A was used for determination of D-gluconic acid and δ-gluconolactone concentrations. The system was fitted with a steel column (250 × 4 mm) packed with a HEMA-BIO 1000 DEAE 10 μm stationary phase (Tessek Ltd., Praha, Czech Republic) using phosphate buffer (0.05 mM, pH 7) at an applied flow rate of 0.5 ml·min1 as the mobile phase with the UV-VIS detector operated at 210 nm. Elution time for the δ-gluconolactone and D-gluconic acid (as a calcium sah) were 4.26–4.29 min or 5.46–5.59 min, respectively. Concentrations of D-glucose were determined on a Preparative Chromatography System WATERS Delta Prep 3000 (Waters, Milford, Massachusetts, USA) using a 300 × 8 mm WATREX Polymer IEX Pb column with RI detection (Waters R 403) using the same mobile phase at a flow rate of 1 ml·min1 at 80°C.
Measurement of Dissolved Oxygen Concentration and Viscosity of the PA Solutions
The concentration of dissolved oxygen in aqueous reaction mixtures was determined at 30°C and 100 kPa pressure using an oxygen meter SevenGo Pro (Mettler Toledo GmbH, Scherzenbach). The dynamic viscosities of the PA solutions with and without oxygen carriers were determined at 25°C using a programmable viscosimeter (Brookfield model LVDV-II+) with a small sample adapter and a spindle SC4-18 (Middleboro, MA, USA) at a shear rate from 1.85 to 22.44 s−1.
RESULTS AND DISCUSSION
Coencapsulation of Oxygen Carriers with GOD in SA-CS/PMCG Capsules
The addition of coencapsulated oxygen carriers at the concentrations of a few percents (w/w) to the PA solution may significantly alter preparation of the SA-CS/PMCG capsules. The requirements for successful coencapsulation of oxygen carriers within GOD-SA-CS/PMCG capsules are as follows: (i) preparation of a stable PA solution; (ii) achievement of a stable PA droplet formation during encapsulation; and (iii) preparation of stable capsules.
Mixing of water-soluble PDMS with the PA solution containing GOD resulted in formation of stable solutions that were capable of forming functional capsules with PDMS concentration of up to 4% (w/w). Heterogeneous emulsion-like systems were formed when PDMS concentrations in the PA solution were higher than 4% (w/w), which resulted in irregular PA droplet formation, thus disabling the encapsulation process. In order to examine water-insoluble oxygen carriers, emulsification of DOD and PFD with the PA solution containing GOD using the conventional emulsifier Pluronic F-68 was tested (concentrations in the range from 1% to 5% w/v). These reactions resulted in the production of unstable emulsions, with a resulting inability to carry out encapsulation. It was noted, however, that the mixing of PDMS with the PA solution containing GOD and PFD or DOD in proper ratios resulted in the production of homogenous and stable emulsions that could be used for a reproducible production of stable and functional GOD-SA-CS/PMCG capsules. It is known that silicone copolymers, such as PDMS, can either themselves be used as emulsifiers or act as the dispersed phase in emulsions [Citation18]. Moreover, PDMS droplets possess colloidal stability in the absence of added stabilisers [Citation19]. Thus, the surface activity of PDMS enables the stabilization of the water-insoluble oxygen carriers DOD and PFD in the SA-CS solution leading to the stable production of the droplets and SA-CS/PMCG capsules. In this context, PDMS is used not only as the oxygen carrier but also as the emulsifier for the water-insoluble substances to be coencapsulated in the polar aqueous environment of the capsules such as SA-CS/PMCG.
The viscosity of the PA solutions appears to further stabilize the resulting emulsions of the PA solution containing 4% (w/w) of PDMS and either 10% (w/w) of DOD or 10% (w/w) of PFD. Addition of PDMS increased the PA solution viscosity by 20% whereas the presence of water-insoluble oxygen carriers DOD or PFD in the PA solutions containing GOD and PDMS decreased the viscosity of PA solutions by 70% and 30%, respectively (). Nevertheless, ability of the PA solutions to form droplets and subsequently stable capsules does not appear to be hampered by the presence of these additional components in the SA-CS solution.
Table 1. Viscosity of the PA solutions and statistical analysis of the size and membrane thickness of GOD-SA-CS/PMCG capsules with coencapsulated oxygen carriers: 4% (w/w) of poly(dimethylsiloxane) (PDMS), 10% (w/w) of n-dodecane (DOD) and 10% of perfluorodecaline (PFD), respectively
shows representative images of GOD-SA-CS/PMCG capsules with and without coencapsulated oxygen carriers. As shown in , the microcapsules were uniform and similar in size and membrane thickness to those reported in our previous work [Citation11]. Despite rather high concentrations of PDMS (4%) and DOD (10%) or PFD (10%) used, the capsules were mechanically and chemically stable even when exposed to biooxidative conditions in a batch-wise reactor or when they were stored in 0.9% (w/v) NaCl at 4°C.
Figure 2. Representative images of GOD-SA-CS/PMCG microcapsules: a) reference sample without oxygen carriers; b) capsules with an emulsion of PDMS 4% (w/w) and PFD 10% (w/w); c) capsules with PDMS 4% (w/w); d) capsules with an emulsion of PDMS 4% (w/w) and DOD 10% (w/w). Digitized images were taken either by an optical microscope (a, c) or a stereomicroscope (b, d) equipped with a digital camera and interfaced to a PC.
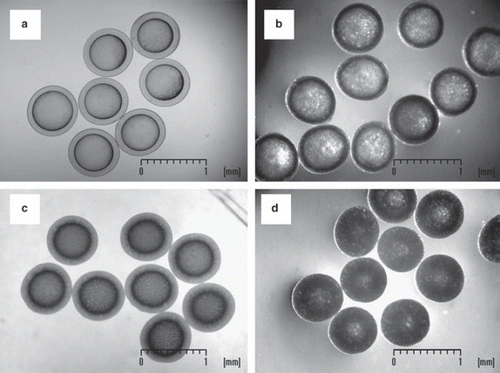
Statistical analysis revealed reproducible preparation of the capsules with SD up to 5% for capsule size or 14% for membrane thickness (). It was not possible to measure membrane thickness of the capsules containing PDMS-DOD due to the high turbidity of the capsules. Batch to batch variation of the capsule size up to 8% is another advantageous feature of the encapsulation protocol used, which enables a reliable comparison of GOD catalytic performance using these different oxygen-carrying systems.
Catalytic Efficiency of Coencapsulated GOD with Oxygen Carriers or with MnO2
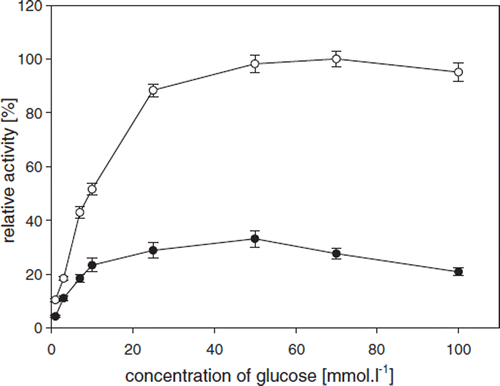
Biooxidation of ß-D-glucose by oxygen via catalysis of GOD (which is usually accompanied by catalase) results in the formation of δ-D-gluconolactone, which is followed by its spontaneous hydrolysis to D-gluconic acid and hydrogen peroxide. Hydrogen peroxide is then split into water and oxygen with the aid of catalase [Citation20]. In this context, optimization of conditions for glucose biooxidation is the first step for successful testing of coencapsulated GOD and oxygen carriers. describes the evolution of relative enzyme activities for GOD-SA-CS/PMCG capsules during ß-D-glucose biooxidations using different glucose concentrations and oxygenated with air or pure oxygen. Enzyme activity (A) was measured spectrophotometrically via the horse-radish peroxidase method described above. The value of A = 21.0±l.l U·g−1 corresponds to 100% activity. The maximum enzyme activities were achieved using a glucose concentration between 50 and 100 mmol·l−1 and using pure oxygen for maximum oxygen saturation. Purging the solution with pure oxygen provided almost three-fold higher oxygen concentration of 0.625 mM compared to 0.25 mM achieved by aeration with air. The latter value may represent a limiting factor, since it corresponds to the K value for oxygen of 0.25 mM [Citation2]. Thus, utilization of pure oxygen may reduce rate-limitation factors during biooxidations catalyzed by encapsulated GOD. Concentration of 70 mmol·l1 of D-glucose was selected for subsequent assessment of biooxidation using GOD and coencapsulated oxygen carriers.
Figure 3. Relative enzyme activities for GOD-SA-CS/PMCG capsules using different oxygen feeding conditions, including air (•) and pure oxygen (○), at different glucose concentrations. Oxygen concentrations in reaction mixtures, measured by an oximeter, were 0.25 mM using air and 0.625 mM using pure oxygen. GOD activities were measured spectrophotometrically. 100% activity = 21.1±1.1 U·g−1.
The set of data demonstrating the effect of coencapsulated oxygen carriers with GOD is shown in . Enzyme activities (A), measured spectrophotometrically, and space-time yields (STY) of the product (mixture of δ-D-gluconolactone and D-gluconic acid), measured by HPLC, were significantly higher in all preparations containing oxygen carriers compared to the reference sample without carriers. In fhis regard, the highest values of enzyme activity of A=38.4±2.0 U·g∼1 and STY of 834±3.4 g·L1day∼1 was observed using the capsules with emulsified DOD and PDMS, which was roughly twofold compared to the reference sample in the absence of oxygen carriers (A=21.0 ±1.1 U·g−1 and STY=44.3±2.0 g·H·day−1 ). It can be concluded there is no significant difference among all systems tested using various oxygen carriers in the biooxidation capability.
Table 2. Comparison of enzyme activity (A) and space-time yield (STY) of the product (mixture of D-gluconic acid and δ-gluconolactone) for GOD-SA-CS/PMCG capsules with the following concentration of coencapsulated oxygen carriers: 4% (w/w) of poly(dimethylsiloxane) (PDMS), 10% (w/w) of n-dodecane (DOD) and 10% (w/w) of perfluorodecaline (PFD), respectively; oxygen concentration was kept at 0.625 mM
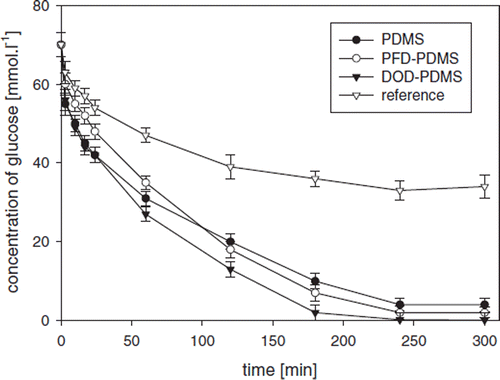
shows the effect of oxygen carriers on product formation in time (mixture of δ-D-gluconolactone and D-gluconic acid, both measured by HPLC) during glucose oxidation. An increase in the product concentration during oxidation () is proportional to glucose consumption as monitored by HPLC and online by a flow micro-calorimeter (), which represents an important consistency test. Thus, an enzyme thermistor [Citation17] can be suggested as a powerful tool for an on-line glucose monitoring in SA-CS/PMCG capsules with encapsulated GOD. The product concentration in the systems with GOD coencapsulated with oxygen carriers was significantly higher compared to the capsules with GOD encapsulated without the oxygen carriers (). No adverse effect of the selected oxygen carriers on the product () and glucose concentrations () was observed. In this regard, observed increase of GOD activity and STY of the product can be explained by increased oxygen solubility within GOD-SA-CS/PMCG capsules due to the presence of oxygen carriers when compared to the reference capsules without carriers.
Figure 4. Effect of coencapsulated oxygen carriers within the GOD-SA-CS/PMCG capsules expressed as the time evolution of the product (D-gluconic acid – δ-gluconolactone mixture) concentration during glucose oxidation. The oxygen carrier concentrations in PA solution were PDMS 4% (w/w), PFD 10% (w/w), DOD 10% (w/w). GOD-SA-CS/PMCG capsules formed in the absence of oxygen carriers were used as the reference. Product concentrations were determined by HPLC. Oxygen concentration was 0.625 mM.
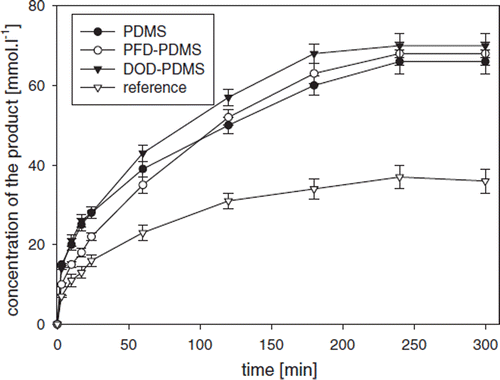
Figure 5. Effect of coencapsulated oxygen carriers within the GOD-SA-CS/PMCG capsules expressed as the time evolution of the glucose concentration monitored on-line by flow microcalorimetry (FC) and sample analysis by HPLC. Depicted concentrations represent average values from measurements by FC and HPLC. The oxygen carrier concentrations in PA solution were PDMS 4% (w/w), PFD 10% (w/w), DOD 10% (w/w). GOD-SA-CS/PMCG capsules formed in the absence of oxygen carriers were used as reference. Oxygen concentration was 0.625 mM.
The increased oxygen solubility within the capsules containing water-soluble PDMS can be explained by an increase in oxygen solubility in PDMS, which is approximately 45- to 50-fold higher than in water [Citation7]. The oil-in-water emulsions formed within the capsules by PDMS, either alone or in combination with DOD or PFD, were used to increase the hydrophobicity and, therefore, the solubility of nonpolar oxygen inside the capsules. It was found that PDMS acts not only as the oxygen carrier but also stabilizes hydrophobic DOD and PFD in the PA solution. PDMS thus can be viewed as a replacement for oxygen-inactive emulsifiers such us Pluronic F-68. PDMS as a non-toxic matrix [Citation7] is also a promising alternative to Pluronic F-68, which is toxic to cells [Citation13], for the encapsulation of whole cell oxidative biocatalysts.
This work demonstrates that there is no synergistic effect of PDMS coencapsulated with hydrophobic DOD or PFD on enhanced GOD activity. Thus, it can be concluded that the use of additional hydrophobic oxygen carriers (DOD or PFD) besides PDMS is not needed, which greatly simplifies the GOD encapsulation protocol. On the other hand, it should be mentioned that there could be an advantage to using coencapsulated emulsions of PDMS with DOD or PFD, mainly in preventing PDMS from being released from the capsules in long-term applications. The 27.9 kDa size of the PDMS macromolecules used may coincide with the molecular weight cut-off of SA-CS/PMCG microcapsules. This cut-off was determined to be around 28 kDa (viscosity radius ∼4.2 nm) by an inverse size-exclusion chromatography using pullulans standards [Citation12]. The size of the statistical coil of pullulans and the PDMS used cannot be a priori considered the same, due to very different chemical structure. Therefore, no direct conclusion can be made on whether PDMS will or will not be released from the capsules. Nevertheless, it can be expected that emulsion droplets (in the expected range of at least tens of nanometers) of PDMS and DOD or PFD will significantly reduce the probability of PDMS being released from the capsules.
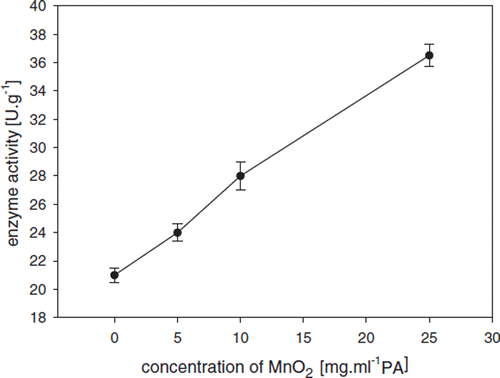
In addition to the above discussed oxygen carriers, MnO 2 was also coencapsulated with GOD in this work, in order to investigate a different mechanism how to improve oxygen level in GOD-SA-CS/PMCG capsules. The expected enhancement of the GOD activity is based on the MnO2 catalyzed decomposition of hydrogen peroxide to oxygen, which (i) partially recovers the oxygen concentration as a reaction rate limiting substrate, and (ii) removes hydrogen peroxide from the reaction mixture to prevent enzyme inactivation [Citation2]. A positive effect of MnO2 coencapsulation within GOD-SA-CS/PMCG capsules on GOD activity is depicted in . An increase in the GOD activity (measured spectrophotometrically) was directly proportional to the MnO2 concentration used with a maximum activity of 36.5 U·g–1 achieved at a MnO2 concentration of 25 mg·ml–1 PA and oxygen concentration of 0.625 mM. This result is similar to that shown in for coencapsulated oxygen carriers. Potential interference of catalase present in the GOD preparation on hydrogen peroxide decomposition can be ignored due to presence of only a trace amount of CAT (GOD/CAT ratio of 18,440). The attempts to work with concentrations of MnO2 higher than 25 mg·ml−1 failed due to clogging of the air-stripping nozzle during the encapsulation process. Nevertheless, the obtained data already demonstrate that MnO2 coencapsulated in GOD-SA-CS/PMCG capsules represents an effective way to optimize this biooxidative process. Additional goals of the study include more in-depth investigations regarding the impact of immobilized oxygen carriers on the physico-chemical properties of capsules (e.g., mechanical and chemical stability, molecular weight cut-off) and their impact on the viability of whole-cell oxidative biocatalysts.
CONCLUSIONS
This work was aimed to enhance the activity and product yield of encapsulated GOD in SA-CS/PMCG capsules by optimization of the oxygen supply protocol. This was done by coencapsulation of oxygen carriers – poly(dimethylsiloxane) (PDMS), PDMS with n-dodecane (DOD) and PDMS with perfluorodecaline (PFD) – together with GOD, in order to increase the oxygen concentration within capsules. Moreover, coencapsulation of MnO 2 with GOD in order to suppress the adverse effect of the produced hydrogen peroxide was studied. A broad set of experimental techniques was employed to analyze the GOD activity and space-time yield of the product (mixture of D-gluconic acid and δ-gluconolactone) during glucose oxidation.
In all systems tested, a significant increase in GOD activity was achieved compared to GOD encapsulated in the absence of the oxygen carriers. Clearly, the utilization of coencapsulated oxygen carriers and MnO 2 represents a promising strategy for improving the catalytic efficiency of other oxygen limited oxidative biocatalysts. PDMS acts both as the oxygen carrier and as an emulsifier for DOD and PFD. In addition, these compounds may stabilize PDMS inside the capsules in the form of emulsion droplets. Coencapsulation of MnO 2 with GOD is recommended as a feasible way for improving the GOD catalytic efficiency, since its presence significantly increases GOD activity and product yield by partial recovery of oxygen, which is a reaction rate limiting substrate.
NOMENCLATURE
In the follow-up work, innovative approaches are needed for direct measurement of the oxygen concentration within the submillimeter GOD-SA-CS/PMCG capsules for more rigorous investigations regarding oxygen mass transfer.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- De Muynck, C., Pereira, C.S.S., Naessens, M., Parmentier, S., Soetaert, W., Vandamme, E.J. (2007). The genus Gluconobacter oxydans: Comprehensive overview of biochemistry and biotechnological applications. Critical Reviews in Biotechnology 27:147–171.
- Vikartovská, A., Bučko, M., Mislovičová, D., Pätoprstý, V., Lacík, I., Gemeiner, P. (2007). Improvement of the stability of glucose oxidase via encapsulation in sodium alginate-cellulose sulfate-poly(methylene-co-guanidine) capsules. Enzyme and Microbial Technology 41:748–755.
- Rosenberg, M., Švitel, J., Šturdík, E., Rosenbergová, I. (1992). Gluconic acid production by Aspergillus niger with the oxygen supply by hydrogen peroxide. Bioprocess Engineering 7:309–313.
- Vorlop, K.-D., Jarzombek, P., Baatz, C., Thielecke, N., Pruesse, U. (2007). Immobilised synzyme-like gold nanoparticles for the oxidation of sugars to sugar acids. 15th International Workshop on Bioencapsulation, 2007, Vienna, lecture O6-1. September 6-8, 2007; publisher: Wien: Universität Wien, 1-4 p.
- Fonade, C., Goma, G., Lafforgue, C. (1988). A new approach of momentum and mass transfer in high cell concentration biological processes. Second International Conference on Bioreactor Fluid Dynamics, publisher: Elsevier Applied Science (London, New York), 309–318
- Vikartovská-Welwardová, A., Michalková, E., Gemeiner, P., Welward, L. (1999). Stabilization of D-amino-acid oxidase from Trigonopsis variabilis by manganese dioxide. Folia Microbiologica 44:380–384.
- Dey, E.S., Norrlöw, O., Liu, Y. (2004). Artificial carrier for oxygen supply in biological systems. Applied Microbiology and Biotechnology 64:187–191.
- Schneider, M., Reymond, F., Marison, I.W., von Stockar, U. (1995). Bubble-free oxygenation by means of hydrophobic porous membranes. Enzyme and Microbial Technology 17:839–847.
- Mateo, C., Palomo, J.M., Fernandez-Lorente, G., Guisan, J.M., Fernandez-Lafuente, R. (2007). Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme and Microbial Technology 40:1451–1463.
- Lacík, I. 2006. Polymer chemistry in diabetes treatment by encapsulated islets of Langerhans: review to 2006. Australian Journal of Chemistry 59:508–524.
- Bucko, M., Vikartovská, A., Lacík, I., Kolláriková, G., Gemeiner, P., Pätoprstý, V., Brygin, M. (2005). Immobiliza-tion of a whole-cell epoxide-hydrolyzing biocatalyst in sodium alginate-cellulose sulfate-poly(methylene-co-guanidine) capsules using a controlled encapsulation process. Enzyme and Microbial Technology 36:118–126.
- Bučko, M., Vikartovská, A., Gemeiner, P., Lacík, I., Kolláriková, G., Marison, I.W. (2006). Nocardia tartaricans cells immobilized in sodium alginate-cellulose sulfate-poly(methylene-co-guanidine) capsules: mechanical resistance and operational stability. Journal of Chemical Technology and Biotechnology 81:500–504.
- Khattak, S.F., Chin, K.S., Bhatia, S.R., Roberts, S.C. (2007). Enhancing oxygen tension and cellular function in alginate cell encapsulation devices through the use of perfluorocarbons. Biotechnology and Bioengineering 96:156–166.
- Kim, H.J., Kim, J.H., Shin, Ch.S. (1999). Conversion of D-sorbitol to L-sorbose by Gluconobacter suboxydans cells co-immobilized with oxygen-carriers in alginate beads. Process Biochemistry 35:243–248.
- Anilkumar, A.V., Lacík, I., Wang, T.G. (2001). A novel reactor for making uniform capsules. Biotechnology and Bioengineering 75:581–589.
- Štefuca, V., Welwardová, A., Gemeiner, P. (1997). Flow microcalorimeter auto-calibration for the analysis of immobilized enzyme kinetics. Analytica Chimica Acta 355:63–67.
- Mislovicová, D., Michálková, E., Vikartovská, A. (2007). Immobilized glucose oxidase on different supports for biotransformation removal of glucose from oligosaccharide mixtures. Process Biochemistry 42:704–709.
- Somasundran, P., Mehta, S.C., Purohit, P. Silicone emulsions. Advances in Colloid Interface 128–130:103–109.
- Prestidge, C.A., Simovic, S. (2006). Nanoparticle encapsulation of emulsion droplets. International Journal Pharmaceutics 324:92–100.
- Blandino, A., Macías, M., Cantero, D. (2002). Modelling and simulation of a bienzymatic reaction system co-immobilised within hydrogel-membrane liquid-core capsules. Enzyme and Microbial Technology 31:556–565.