Abstract:
Background: The binding of anti-acetylcholine receptor antibodies (AChRAb) to the main immunogenic region (MIR) of AChR α-subunit in the neuromuscular junction is the major pathogenesis of myasthenia gravis (MG). Methods: A synthetic peptide of 10 amino acids corresponding to the MIR of human AChR was coupled with cellulose beads to make an antigen-specific immunoadsorbent (hMIR10-CB). Results: The hMIR10-CB could remove AChRAb in MG sera by 40.3±2.3%, compared to a tryptophan nonspecific adsorbent Trp-CB by only 22.4±1.5% as determined in ELISA, and also showed good blood compatibility for blood cells, plasma ions and plasma proteins as checked in whole blood perfusion in rabbits. Conclusions: The antigen-specific immunoadsorbent hMIR10-CB can serve as a potential candidate in the immunoadsorption treatment of MG.
INTRODUCTION
Myasthenia gravis (MG) is an organ-specific autoimmune disease mediated mainly by anti-acetylcholine receptor (AChR) antibody (AChRAb). The binding of AChRAb to the AChR on the membrane in neuromuscular junction results in a decrease of AChR number, leading to skeletal muscle weakness and fatigability. Chemically, AChR consist of 5 homologous subunits in the stoichiometry ααβγδ or αβϵδ. It has been found that the AChRAb can be detected in 85-90% of MG patients [Citation16]. However, approximately 65% of the AChRAb, directed against the main immunogenic region (MIR), referring to the residues 67-76 of α-subunit of AChR, is highly pathogenic [Citation15]. Therefore, the pathogenic anti-MIR AChRAb play the key role in pathogenesis of MG.
The ideal immunosuppressive therapy for MG is to eliminate the autoimmune response to AChR without affecting the immune response to other antigens. Nonspecific immunosuppressive therapies such as immunosuppressants, thymectomy, plasmapheresis, and intravenous administration of human immunoglobulin [Citation3] can control symptoms of MG and greatly reduce its mortality. However, effects related to these therapeutic methods are also seen. Several specific immunosuppressive therapies have been investigated in the animal models of MG for the past years. More recently, many tests on the specific immunosuppression of MG have been focused on the autoreactive T cells or B cells in the immune responses leading to the pathogenesis of MG. These include the use of monoclonal antibodies directed against CD molecule to damage immune cells [Citation11], the use of cytokines to suppress dentritic cells (DCs) [Citation7], and the use of some agents to activate CD4+CD25+ regulatory T cells (Treg) [Citation1, Citation2].
One of the choices for specific immunological therapy of MG is to make an immunoadsorbent to remove pathogenic AChRAb. In this study, a synthesized peptide of 10 amino acids corresponding to the MIR of α-subunit of human AChR as ligands was coupled with the cellulose beads as carriers to construct an antigen-specific immunoadsorbent. The antigen-specific immunoadsorbent was tested for the removal efficiency of AChRAb in the sera of MG patients, and for the blood compatibility in rabbits.
MATERIALS AND METHODS
Preparation of the Immunoadsorbent hMIR10-CB
A peptide of 10 amino acids (WNPDDYGGVK) corresponding to the MIR at residues 67–76 of α-subunit of human AChR was synthesized as ligands. 10% cellulose beads were prepared by the method of suspension regeneration, and activated as carriers using epichlorohydrin [Citation12]. The synthesized peptide was then coupled with the cellulose beads to make an antigen-specific immunoadsorbent (hMIR10-CB). The synthesized peptide was immobilized on the cellulose beads by incubation overnight at 40°C with shaking. The immobilization was washed with distilled water. The blocking of any remaining active groups was carried out by incubation for 2 h at room temperature in 2 volume of 1 M ethanolamine, and washed once again until the solution of immunoadsorbent reached neutralization. The prepared immunoadsorbent hMIR 10-CB was stored at 4°C . In parallel, tryptophan was immobilized on the same amount of cellulose beads under the same condition as a control (Trp-CB).
Immunoadsorption of AChRAb by hMIR10-CB
Three MG sera were obtained from the patients with AChRAb-positive in the Affiliated Hospital of Yanbian University, China. Two of them were diagnosed as MG clinical class IIa, and one as IIb based on the Osserman clinical classification. The mixed sera were prepared from each patient with equal volume just before the experiment. A 1.5 ml of mixed sera above was incubated with 0.5 ml (approximately 0.3 g) of hMIR10-CB or Trp-CB for 2 h at 37°C with shaking (140 r/min). Similarly, a 1.5 ml of the same sera was incubated with 0.5 ml PBS buffer as control.
ELISA Analysis for the Immunoadsorption of hMIR10-CB
ELISA was used to check the removal efficiency of AChRAb from MG sera by hMIR10-CB as previously described [Citation17]. 96-well microtiter plate was coated with α-bungarotoxin (α-BT) in carbonate-bicarbonate buffer for 2 h at 37°C, and then blocked with 10% FCS overnight at 4°C. A crude extract of AChR from human muscle was added by incubation for 2 h at 37°C. After further incubation with 100 μl of serum for 2 h at 37°C , the color reaction was developed by addition of HRP-conjugated goat anti-human IgG. The adsorption rate was as follows: Adsorption rate = (1- value A after adsorption / value A before adsorption) × 100 %.
Blood Compatibility of hMIR10-CB
The blood compatibility of hMIR10-CB was determined in vivo in rabbits by whole blood perfusion extracorporeal circulation. Twelve healthy rabbits were randomly divided evenly into 3 groups. The rabbits were treated with immunoadsorbent hMIR10-CB in group 1, Try-CB in group 2, and cellulose beads alone as control in group 3. All the equipments and reagents used in the whole blood perfusion were autoclaved for 20 min at 1.05 kg/cm2 and heparinized in 3 u/ml of heparin solution overnight at room temperature. The extracorporeal circulation was performed from rabbit internal carotid artery, through the column containing hMIR10-CB (or Trp-CB, or cellulose beads alone) in 39.4 °C water incubator, to the pump, and finally back to the rabbit external jugular vein. The whole blood perfusion was cycled for 2 h at a speed of 6 ml/min. Blood samples were collected before and after the circulation. All the samples underwent the determination of blood cells, plasma ions, and plasma proteins.
RESULTS
Removal Efficiency of AChRAb by hMIR10-CB
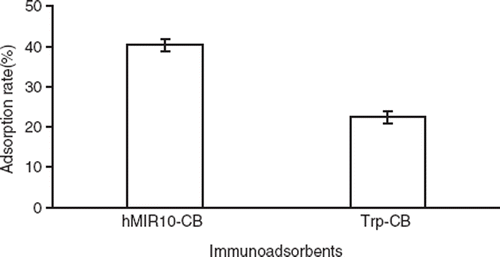
For calculation and statistical analysis, the value A after adsorption in hMIR10-CB or Trp-CB tube was corrected with the value A after adsorption in PBS control tube to remove any affects of antibody natural decay. The adsorption rate of hMIR10-CB to AChRAb (40.3±2.3%) was significantly higher than that of Trp-CB (22.4±1.5%) as determined in ELISA (Value A before adsorption: 1.067±0.102, value A after adsorption in hMIR10-CB: 0.636±0.151, value A after adsorption in Trp-CB: 0.828±0.133). The Trp-CB also showed a removal effects on AChRAb as compared with control (see ).
Figure 1. Adsorption rates of anti-acetylcholine receptor antibodies (AChRAb) in the sera of patients with myasthenia gravis (MG) by the immunoadsorbent hMIR10-CB made by conjugation of 10 amino acids referring to the MIR at residues 67–76 of (α-subunit of human AChR and cellulose beads, and Trp-CB made by conjugation of tryptophan and cellulose beads.
Blood Compatibility of hMIR10-CB
There was no significant difference for the cellulose beads control group in blood cells, plasma ions, and plasma proteins before and after adsorption (data not shown). Furthermore, no significant difference was found in blood cells and plasma ions before and after adsorption for both hMIR10-CB and Trp-CB group (see ). However, plasma total proteins, plasma albumins, and plasma globulins were all significantly decreased in hMIR10-CB group and Trp-CB group before and after the experiment, but there was not a significant difference between hMIR10-CB group and Trp-CB group (see ).
Table 1. The effects of immunoadsorbent hMIR10-CB on blood cells and plasma ions
Table 2. The changes in plasma proteins before and after adsorptions of immunoadsorbents hMIR10-CB and Trp-CB (mean± s, n = 5)
DISCUSSION
The treatment of removing pathogenic antibodies has lasted for almost 40 years. Plasmapheresis is an efficient short-term treatment of MG, especially for myasthenic crisis. However, its drawback of removing any other serum important molecules is also serious. Adsorption columns containing tryptophan [Citation10] or staphylococcal protein A (SPA) [Citation9] remove not only pathogenic antibodies, but also some protective antibodies. A specific immunoadsorption for AChRAb was first introduced in 1996 by Takamori et al. [Citation13, Citation14]. They constructed an immunoadsorbent using a synthesized peptide referring to residues 183–200 amino acids of α-subunit of Torpedo AChR as ligand. This was unlikely to be efficient since only a small amount of AChRAb in MG patient sera was directed against that epitope [Citation6]. In 2005, Psaridi-Linardaki et al. [Citation8] and Guo et al. [Citation5] developed, respectively, a new immunoadsorbent in which the same ligand of expressed extracellular domain of α-subunit 1–210 of human AChR was used, but with different carriers. The adsorption capacity of the prepared immunoadsorbents was demonstrated to be efficient. However, the fragment of α-subunit 1–210 is big enough and able to act as immunogen to trigger the pathogenesis of MG in sera if it is released from the immunoadsorbents of the column, although it was proved not to do so in the case of Psaridi-Linardaki’s experiment. In 2008, Zisimopoulou et al. [Citation18] expressed the extracellular domains of all other subunits of human AChR in E coli and used them as ligands in preparation of immunoadsorbents. It may not be useful in clinical application in the treatment of MG patients since AChRAb directed against other subunits of AChR except α are probably not pathogenic [Citation4].
Removing pathogenic AChRAb was the focus of this study. We selected the MIR 10 peptides of human AChR as ligand in making specific immunoadsorbent because the MIR against which a majority of AChRAb is directed is the major target of pathogenic AChRAb both in man and in experimental animals. Furthermore, the synthetic 10 amino acid of MIR is small, and possibly not potentially immunogenic in the case of being released from the column. The fragment of MIR 10 amino acids itself was able to fold a natural conformation as epitope so that the AChRAb could recognize it [Citation15]. Therefore, the use of hMIR10-CB as an antigen-specific immunoadsorbent may serve as an efficient treatment in removing pathogenic AChRAb for MG patients, while eliminating the worry of its acting as immunogen to induce MG.
In this paper, we prepared an AChR-specific immunoadsorbent (hMIR10-CB) by immobilizing a synthetic 10 amino acid of MIR of human AChR α-subunit as ligand on the cellulose beads as carrier. In the MG serum immunoadsorption experiment, the hMIR10-CB was able to remove AChRAb by 40.3±2.3%, whereas the nonspecific control adsorbent Trp-CB (tryptophan as ligand) eliminated AChRAb by only 22.4±1.5% as checked in ELISA (see ), indicating that removal capacity of the antigen-specific immunoadsorbent was better than that of the nonspecific adsorbent. Furthermore, in the whole blood perfusion extracorporeal circulation experiment in rabbits, the hMIR10-CB had neither effect on blood cells nor on plasma ions (see ), suggesting that it has good blood compatibility. Meanwhile, the hMIR10-CB reduced plasma proteins. However, the reduction of plasma proteins was not statistically significant as compared with that of Trp-CB (see ), demonstrating that the affinity adsorption of hMIR10-CB to AChRAb was specific.
hMIR10-CB is a good candidate and holds promise for the specific immunoadsorption of AChRAb, especially of pathogenic AChRAb in MG treatment.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Aricha, R., Feferman, T., Fuchs, S., Souroujon, M.C. (2008). Ex vivo generated regulatory T cells modulate experimental autoimmune myasthenia gravis. J Immunol 180: 2132–2139.
- Aruna, B.V., Sela, M., Mozes, E. (2006). Down-regulation of T cell responses to AChR and reversal of EAMG manifestations in mice by a dual altered peptide ligand via induction of CD4+ CD25+ regulatory cells. J Neuroimmunol 177: 63–75.
- Gold, R., Schneider-Gold, C. (2008). Current and future standards in treatment of myasthenia gravis. Neurotherapeutics 5(4): 535–541.
- Graus, Y., Meng, F., Vincent, A., van Breda Vriesman, P., de Baets, M. (1995). Sequence analysis of anti-AChR antibodies in experimental autoimmune myasthenia gravis. J Immunol 154: 6382–6396.
- Guo, C.Y., Li, Z.Y., Xu, M.Q., Yuan, J.M. (2005). Preparation of an immunoadsorbent coupled with a recombinant antigen to remove anti-acetylcholine receptor antibodies in abnormal serum. J Immunol Methods 303: 142–147.
- Lefvert, A.K., Cuenoud, S., Fulpius, B.W. (1981). Binding properties and subclass distribution of anti-acetylcholine receptor antibodies in myasthenia gravis. J Neuroimmunol 1: 125–135.
- Meriggioli, M.N., Sheng, J.R., Li, L., Prabhakar, B.S. (2008). Strategies for treating autoimmunity: novel insights from experimental myasthenia gravis. Ann N Y Acad Sci 1132: 276–282.
- Psaridi-Linardaki, L., Trakas, N., Mamalaki, A., Tzartos, S.J. (2005). Specific immunoadsorption of the autoantibodies from myasthenic patients using the extracellular domain of the human muscle acetylcholine receptor α-subunit. Development of an antigen-specific therapeutic strategy. J Neuroimmunol 159: 183–191.
- Schneidewind-Muller, J.M., Winkler, R.E., Tiess, M., Muller, W., Ramlow, W. (2002). Changes in lymphocytic cluster distribution during extracorporeal immunoadsorption. Artif Organs 26(2): 140–144.
- Splendiani, G., Cipriani, S., Passalacqua, S., Stur, A., Costanzi, S., Fulignati, P., Staffolani, E., Casciani, C.U. (2003). Plasmaperfusion on tryptophan columns can improve the clinical outcome of patients affected with myasthenia gravis. Artif Cells Blood Substit Immobil Biotechnol 31(1): 69–79.
- Stieglbauer, K., Topakian, R., Schaffer, V., Aichner, F.T. (2009). Rituximab for myasthenia gravis: Three case reports and review of the literature. J Neurol Sci Epub ahead of print.
- Sundberg, L., Porath, J. (1974). Preparation of adsorbent for biospecific affinity chromatography. J Chromatology 90: 87–92.
- Takamori, M., Ide, Y. (1996). Specific removal of anti-acetylcholine receptor antibodies in patients with myasthenia gravis. Transfus Sci 17: 445–453.
- Takamori, M., Maruta, T. (2001). Immunoadsorption in myasthenia gravis based on specific ligands mimicking the immunogenic sites of the acetylcholine receptor. Ther Apher 5(5): 340–350.
- Tzartos, S.J., Kokla, A., Walgrave, S., Conti-Tronconi, B.M. (1998). Localization of the main immunogenic region of the human muscle acetylcholine receptor to residues 67–76 of the α-subunit. Proc Natl Acad Sci USA 85: 2899–2903.
- Vincent, A., Palace, J., Hilton-Jones, D. (2001). Myasthenia gravis. Lancet 357: 2122–2128.
- Yang, L., Cheng, Y., Yan, W.R., Yu, Y.T. (2004). Extracorporeal whole blood immunoadsorption of autoimmune myasthenia gravis by cellulose tryptophan adsorbent. Artif Cells Blood Substit Immobil Biotechnol 32(4): 519–528.
- Zisimopoulou, P., Lagoumintzis, G., Poulas, K., Tzartos, S.J. (2008). Antigen-specific apheresis of human anti-acetylcholine receptor autoantibodies from myasthenia gravis patients’ sera using Escherichia coli-expressed receptor domains. J Neuroimmunol 200: 133–141.