Abstract
Up to 32 Landrace ear marginal tissue samples were used for establishing a fibroblast cell bank by the means of primary explantation and cryopreservation. Biological analysis suggested that the Population Doubling Time (PDT) of the cell line was approximately 24h. The diploid accounted for 97.2% of the whole population; isozyme analysis of Lactic Dehydrogenase (LDH) and Malic Dehydrogenase(MDH) disproved cross-contamination from other cell lines. The results of bacterium, fungus, virus and mycoplasma tests were all negative. The transfection rates of three fluorescent proteins were high, indicating that the exogenous genes could be effectively expressed in the cells. It had not only preserved the precious germplasm resource of the Landrace on the cell level but also provided valuable material for the researches of genomics, postgenomics, somatic cloning and so on.
INTRODUCTION
With the improvement of people's living standard and the development of science and technology, the development of animal husbandry worldwide has emphasized high-yielding breeds, resulting in the extinction of many local ones with poor economic performances. The diversity of animal genetic resources has been confronted with an unprecedented challenge. While some government departments, organizations, and experts have appealed for conservation and management of livestock and poultry genetic resources, there has still been a massive loss. At present, China has a total of 596 local livestock breeds, among which 17 have become extinct, 336 are being threatened at different levels, and only 243 are relatively safe [Citation1]. Genetic resources of domestic animals are on the edge of depletion throughout the earth. Therefore, genetic resource preservation of livestock and poultry cannot be ignored. At present, preservation of individual animals, semen, embryos, genomic libraries and cDNA libraries are all practical options. In addition, modern cloning techniques have made somatic cells an attractive resource for conserving animal genetic materials. Culture centers throughout the world, for example, ATCC, ECACC and DSMZ, have been established, which hold great promise in research scenarios of the realms including genetics, post-genetics transgenic therapy, cell and molecular biology and other unforeseeable applications.
Landrace, originating in Denmark, is characterized with fast growth, high forage utilization ratio, high yield of lean meat, and fecundity. Introduced in the 1960s, its adaptability improved and scope of breeding greatly increased after 10 years of domestication. Landrace could play an important role as a male parent in the improvement of the lean meat rate of local pigs through hybridization for China in the future [Citation2].
In this chapter, the Landrace ear marginal fibroblast line has been successfully constructed, not only for the purpose of long-term preservation of animal genetic resources on the cell level, but also to provide a valuable experimental material for scientific researches of biodiversity. This study inaugurated a new technical line in the preservation and maintenance of domestic animal resources.
MATERIALS AND METHODS
Materials
The 32 ear marginal tissue samples of Landrace in this research were provided by Pig Breeding Farm of The Chinese Academy of Agricultural Sciences, Beijing, PR China. Except where specifically pointed out, all chemicals were obtained from Sigma Chemical Co. (St. Louis, MO).
Isolation and Culture of Landrace Ear Marginal Fibroblasts
Ear marginal tissue samples (about 1 cm2) were acquired from 32 Landrace (18 males and 14 females) and placed into separate tubes containing DMEM supplemented with ampicillin (100 U/ml) and streptomycin (100 g/ml). The samples were washed five times with phosphate buffered saline (PBS) and chopped into 1mm3 pieces, which were subsequently seeded on the surface of a tissue culture flask containing DMEM + 10% fetal bovine serum at 37°C in a humidified incubator with 5% CO2 [Citation3,Citation4].
The media were refreshed when the cells reached 80–90% confluence, and one day later they were passaged with 0.25% trypsin and separated into prepared culture flasks at the ratio of 1:2 or 1:3 [Citation4,Citation5]. All samples were cultured in separate flasks to make sure cells from different individuals would not commingle with those from others, and were treated indiscriminatingly for further evaluation and detection.
Cryogenic Preservation and Recovery
The fibroblasts in the logarithmic phase were enumerated under a hemocytometer, pelleted and resuspended in freezing medium (10% DMSO+40% fetal bovine serum+50 %DMEM) at the concentration of (3–4) ×106 viable cells per milliliter. Cell suspensions were aliquoted into sterile plastic cryovials labeled with breed, species, gender, freezing serial number and the date. The vials were sealed and kept at 4°C for 20 min–30 min to equilibrate the DMSO, put into a programmed cryopreservation system, and then transferred to liquid nitrogen for long-term storage [Citation6].
Estimation of Cell Viability
Viabilities before freezing and after recovery were determined using the Trypan blue exclusion test. The number of non-viable cells was determined in a visual field of 1000 cells [Citation7].
Cell Growth Curve
Cells were seeded in 24-well microplates at the density of approximately 1.5×105 cells per well, cultured for 7 d, and counted every day (3 wells each time) afterwards. The mean cell counts at each time point were then used to plot the growth curve, based on which the PDT was calculated [Citation8–10].
Chromosome Analysis
Chromosomes were prepared, fixed, and stained following the standard methods [Citation11]. Cells were harvested when they reached 80–90% confluence, hypotonic and fixed, and the chromosome numbers were counted from 100 spreads under an oil immersion objective upon Giesma staining. Relative length, arm ratio, and centromeric index and type were calculated according to the protocol of Kawarai et al. [Citation12].
Isoenzyme Analysis
Isoenzyme patterns of lactic dehydrogenase (LDH) and malic dehydrogenase (MDH) were detected using vertical slab non-continuous polyacrylamide gel electrophoresis (PAGE) assay. The cells were harvested, and protein extraction solution (0.9% Triton X-100, 0.06 mmol NaCl:EDTA in mass ratio 1:15) was added into the cell suspension of 5×107 cells/mL according to the method described by Simpson [Citation13]. Then the mixture was centrifuged and the supernatant was stored in aliquots at −80°C. Sucrose solution (40%) and samples were mixed (1:1 (v/v)) and then loaded into the individual lanes of the polyacrylamide gel [Citation14].
Microbial Analysis
Detection of bacteria and fungi. The cells were cultured in DMEM supplemented with 10% fetal bovine serum free of antibiotics and observed for the presence of bacteria and fungi at 3d after subculture according to the method of Doyle et al. [Citation15].
Detection of mycoplasmas. According to the American Type Culture Collection protocol, the cells were cultured in antibiotic-free medium for at least 1 week, and then fixed and stained with Hoechst 33258 [Citation16].
Virus detection. Hay's hemadsorption protocol was used to examine the samples for cytopathogenesis using phase-contrast microscopy [Citation17,Citation18].
Expression of Fluorescent Proteins in Landrace Fibroblastic Cell
According to the methods of Wu et al., three fluorescent proteins (pDsRed1-N1, pEGFP-N3 and pEYFP-N1) were transfected into fibroblasts through plasmid DNA (BD Bioscieces Clontech product, Japan) and Lipofectamine 2000 (Invitrogen, USA). The cultured cells were observed at 24h, 48h, 72h, 96h, 1 week, 2 weeks, and 1 month after transfection. The three fluorescent protein genes were transfected under excitation wavelength of 543 nm, 488 nm, and 488 nm and 543 nm, respectively. In each group 10 visual fields were captured to take pictures and to calculate the transfection efficiencies, which were formulated as the ratio of the positive cell numbers to that of the total under a confocal microscope. Counts from 10 fields were used to calculate the transfection efficiencies [Citation19].
Statistical Analysis
Statistical analysis was conducted with SPSS 13.0 software. It was considered statistically significant when P<0.05.
RESULTS
Morphological Observation of Landrace Fibroblastic Cells
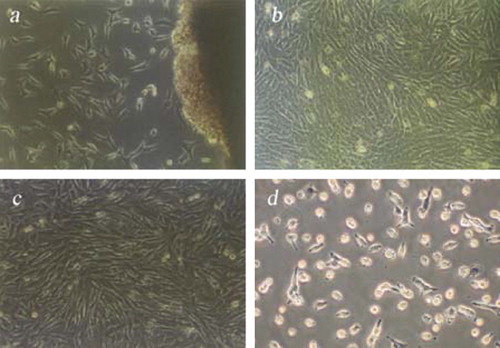
At 5 days after tissue explants adhered to the flask, the cells showed typically fibrous and fusiform morphologies with centrally located oval shaped nuclei. However, there were some exhibiting the shapes of epithelial cells. Cells spread on the bottom of the flasks in eight or ten days and then formed a cell monolayer. Due to their rapid growth, fibroblasts gradually replaced their epithelial counterparts in the subcultures (). After 2–3 passages, a relatively purified fibroblast line was obtained (). The viabilities of Landrace fibroblasts before freezing and after recovery evaluated through Trypan Blue exclusion tests were 98.4±0.22% and 96.8±0.17%, respectively.
Growth Dynamics
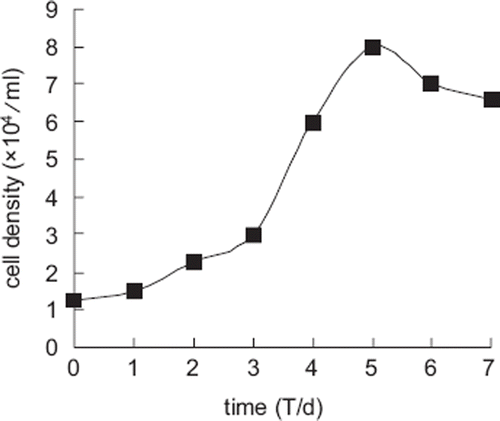
The growth curve of Landrace fibroblasts appeared sigmoidal () and the population doubling time (PDT) was approximately 24 h. There was a lag time or latency phase of about 24 h after seeding, corresponding to the adaptation and recovery of the cells from protease damage, and afterwards the cells proliferated rapidly and entered the exponential phase. As the cell density increased, proliferation receded as a result of contact inhibition and the cells entered the plateau phase after the eighth day.
Karyogram and Chromosome Number of Landrace
In Landrace the chromosome number of the diploid is 2n=38, consisting of 36 autosomes and two sex chromosomes, XY () or XX. The centromere morphology was described as follows:
Figure 3. (a) Chromosomes of Landrace in the metaphase(♂); (b) Karyotype of Landrace fibroblasts(♂).
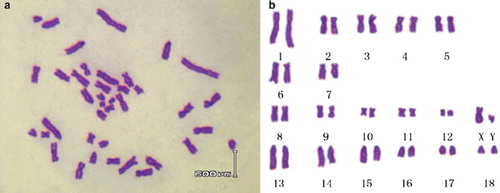
No. 1–5: Submetacentric Chromosome
No. 6–7: Acrocentric Chromosome
No. 8–12: Metacentric Chromosome
No. 13–18: Telocentric Chromosome
The parameters, including relative length, centromere index and kinetochore type, were as shown in .
Table 1. Chromosome Parameters of Landrace (♂)
Isoenzyme Polymorphorism
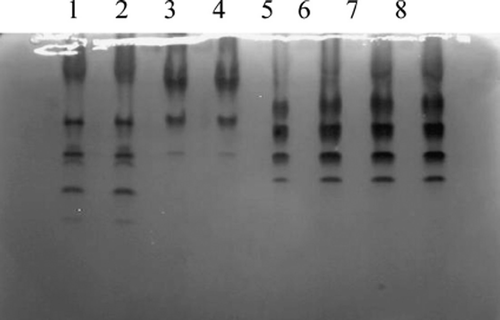
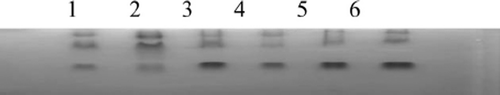
In this study, the isoenzyme patterns of lactic dehydrogenase (LDH) and malic dehydrogenase (MDH) were obtained using vertical slab non-continuous polyacrylamide gel electrophoresis (PAGE), stained by Coomassie brilliant blue and compared with those from other species ( and ). The LDH RFs were in the order of LDH-5, LDH-4, LDH-3, LDH-2, and LDH-1 (), while the two bands of MDH were m-MDH and s-MDH (). The results indicated that there was no cross-contamination from other cell lines.
Microbial Analysis
The medium was clear all the time and no abnormal changes could be observed under the microscope. The results indicated that the Landrace ear marginal fibroblasts were free of bacterial contamination (). Mycoplasma detection suggested that the fibroblasts were free of mycoplasmas. If there was abundant punctiform and filiform blue fluorescence in the nucleoli, it could be concluded that the cells were contaminated by mycoplasmas (), just as the positive result of Li et al. [Citation20]. As is shown by the cytopathogenic evidence and the hemadsorption test, tests for virus contamination were negative.
Expression of Fluorescent Proteins in Landrace Fibroblasts
The three fluorescent genes, pDsRed1-N1, pEGFP-N3 and pEYFP-N1, were all highly expressed according to the optimized condition at 24h, the transfected cells increased gradually as time passed by , and the transfection efficiencies are listed in .
Figure 7. The comparative figures of three fluorescent proteins at 24 h after transfection using a confocal microscope (Nikon TE-2000-E, Japan) with excitation wavelengths of 433 nm-588 nm to determine the transfection efficiencies. (a,d), (b,e) and (c,f) were the transfection results of pDsRed1-N1, pEGFP-N3 and pEYFP-N1, respectively.
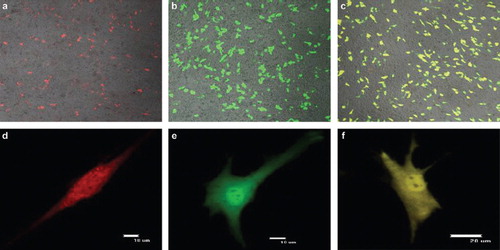
Table 2. The transfection efficiencies of three fluorescent proteins in Landrace
DISCUSSION
In this research, a Landrace ear marginal fibroblast line was established successfully, and was frozen into 30 cryovials within Passage 5 at a density above 3×106 cells/ml. The cultured cells were typical fibroblasts in morphology. The viabilities, although still above 85%, decreased in a certain degree after thawing, indicating an ineluctable injury in the process of cryopreservation. The growth curve of Landrace fibroblasts had an obvious “S” shape, experienced the latent phase, exponential phase, and plateau phase. Initially upon explantation, epithelial cells and fibroblasts would sprout simultaneously from the tissues. Fibroblasts adhered more easily and could be trypsinized more readily, whereas the epithelial cells weren't so Ren [Citation6], according to which distinction the fibroblasts could be purified through serial passage.
Bacteria and fungi grew rapidly, led to nutrients' depletion, and constituted a threat to the culture's survival, but could be detected even with the naked eye. However, it was fairly difficult to perceive mycoplasma contamination, which could coexist with cells for a long term, and the infected cells became slow in growth and proliferation, morphologically irregular, and massively dead. The microbiological detection results suggested that the Landrace fibroblast bank was healthy and free of micoplasma contamination.
Chromosomes were in accordance with those typical pigs and were categorized into four groups, 5SM+2ST+6M+6T, according to their morphology. There was an obvious secondary constriction on Chromosomes 6 and 10 as Zhang et al. [Citation21] reported. However, in this research, possibly related to staining procedure, secondary constriction was easily found in some chromosomes, while seldom observable in others.
Lactate dehydrogenase isoenzyme (LDH) and malate dehydrogenase (MDH), the contents and activities of which were species-specific and relatively conservative during evolution, were used as biochemical indicators in classification, and were hence applied into the identification of cell origin and cross-contamination [Citation22]. Novel detection methods emerged as the development of biological technology, initially from the karyotype analysis, species-specific antigen immunofluorescence techniques, as well as isozyme detection, to the newly emerged DNA fingerprinting analysis and PCR methods after the 1980s [Citation23–27]. However, the polymorphism of biochemical analysis of isozymes was considered as the standard method in the American-type Culture Collection, European Collection of Animal Cell Cultures, and Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH on quality control and cross-contamination detection among cell lines.
The LDH of mammals have two kinds of subunits, H and M and five tetramer compositions, namely H4, H3M, H2M2, HM3 and M4. The isoenzymes are distinct in their catalytic abilities, physical and immunological characteristics. H is a strongly negative-charged subunit, and the motility font of H4 tetramer was the fastest in the gels. Therefore, the H4 tetramer was defined as LDH1, which tranformed lactic acid into acetone acid. That of the M4 tetramer was the slowest corresponding to LDH5 [Citation28], and its function was to catalyse pyruvic acid into lactic acid. MDH was a dimer with two types, s-MDH and m-MDH, and played a vital role in tricarboxylic acid cycle in both eukaryotic cells and prokaryotic cells. It could not only catalyze the oxidation of malic acid into ketosuccinic acid, but also contribute to the reduction reaction of ketosuccinic acid. MDH had different composition in different spices; therefore MDH isozyme detection could display genetic characteristics and facilitate species indentification of cell lines. The motility font of s-MDH is faster than that of m-MDH in the gels.
Exogenous genes were highly expressed in the Landrace cells, with the highest transfection efficiencies at 48h. While the transfection efficiencies decreased, intensive fluorescences were still observed even after a week, indicating that the exogenous genes could be replicated, transcribed, translated, and subsequently modified in the fibroblasts. The results provide a solid theoretical and technical basis for structural genomics, functional genomics, and transgene research concerning the Landrace in the future.
In summary, the Landrace fibroblast bank was successfully established, containing biological normal and genetically stable fibroblasts, all aspects of which had met the standards of cell line quality supervision of major international culture collection and centers. Exogenous gene expression results supported their strong vitality and rationalized their applications in transgenic therapy and genetics. The precious genetic resource of Landrace were effectively preserved at cell level and could serve as a basis and biological materials for genetics, biomedical sciences, cell and molecular biology, immunology, and so on.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Ma, Y.H., Fen, W.Q. (2002). The evaluation of livestock and poultry breed resources. Ecology of Domestic Animal. 3:1–5.
- Xu, G.F., Chen, K.W. (2003). Photograph Album of China Indigenous Poultry Breeds [M], China Agricultural Press, Beijing.
- Freshney, R.I. (2000). Culture of Animal Cells: A Manual of Basic Technique [M], 4th, Wiley-Liss, New York, 49–175.
- Guan, W.J., Ma, Y.H., Zhou, X.Y., Liu, G.L., Liu, X.D. (2005). The establishment of fibroblast cell line and its biological characteristic research in Taihang black goat. Review of China Agricultural Science and Technology. 7:25–33.
- Zhou, X.M., Ma, Y.H., Guan, W.J., Zhao, D.M. (2004). Establishment and identification of Debao pony ear marginal tissue fibroblast cell line. AJAS. 17:1338–1343.
- Ren, F.L., Li, Y., Zhang, Y. (2002). In vitro cultivation and freezing of bovine skin fibroblast cells. Scalper Magazine. 28:8–10.
- Freshney, R.I. (1994). Culture of Animal Cells: A Manual of Basic Technique, 3rd, John Wiley, New York.
- Qi, Y.T., Tu, Y.D., Yang, D., Chen, Q., Xiao, J., Chen, Y.Q. (2007). Cyclin A but not cyclin D1 is essential in c-myc-modulated cell cycle progression. J Cell Physiol. 210:63–71.
- Hirofumi, S., Kentaro, Y., Kouichi, H., Tsuyoshi, F., Norihiro, T., Norio, N. (2006). Efficient establishment of human embryonic stem cell lines and long-term maintenance with stable karyotype by enzymatic bulk passage. BBRC. 345:926–932.
- Weingartl, H.M., Sabara, M., Pasick, J., van Moorlehem, E., Babiuk, L. (2002). Continuous porcine cell lines developed from alveolar macrophages Partial characterization and virus susceptibility. J Viro. Meth. 104:203–216.
- Costa, U.M., Reischak, D., da Silva, J., Ravazzolo, A.P. (2005). Establishment and partial characterization of an ovine synovial membrane cell line obtained by transformation with Simian Virus 40 T antigen. J. Virol. Meth. 128:72–8.
- Sun, Y.L., Lin, C.S., Chou, Y.C. (2006). Establishment and characterization of a spontaneously immortalized porcine mammary epithelial cell line. Cell Biol. Int. 30:970–976.
- Kawarai, S., Hashizaki, K., Kitao, S. (2006). Establishment and characterization of primary canine hepatocellular carcinoma cell lines producing alpha-fetoprotein. Vet. Immunol. Immunop. 113:30–36.
- Simpson, R.J. (2003). Proteins and Proteomics: A Laboratory Manual, Science Press, Beijing.
- Doyle, A., Hay, R. Kirsop, B.E. (1990). Animal Cells: Living Resources for Biotechnology. Cambridge University Press. Cambridge, UK, 81–100.
- Masover, G.K., Becker, F.A. (1998). Detection of mycoplasmas in cell cultures by cultural methods. Miles, R.J., Nicholas, R.A.J., , Methods in Molecular Biology, Vol. 104: Mycoplasma Protocols, Humana Press Inc., Totawa, NJ, 207–15, 217–26.
- Hay, R.I. (1992). Cell line preservation and characterization. Freshney, R.I. Animal Cell Culture: A Practical Approach, 2nd, Oxford University Press, Oxford, 104–135.
- Wu, H.M., Guan, W.J., Li, H., Ma, Y.H. (2008). Establishment and characteristics of white ear lobe chicken embryo fibroblast line and expression of six fluorescent proteins in the cells. Cell Biol. Int. 32:1478–1485.
- Zhou, X.M., Ma, Y.H., Guan, W.J., Wen, J. (2005). Establishment and characteristics of a Beijing fatty chicken embryo fibroblast cell line. Chinese Journal of Animal and Veterinary Sciences. 36:209–215.
- Li, L.F., Yue, H., Ma, J.Z., Guan, W.J., Ma, Y.H. (2009). Establishment and characterization of a fibroblast line from Simmental cattle. Cryobiology. 59:63–68.
- Zhang, Y., Liu, P., Xu, R. (2004). Studies on the karyotype and C-banding of chromosomes of Jianbai Xiang pig. Journal of Mountain Agriculture and Biology. 23(3):215–219.
- Masover, G.K., Becker, F.A. (1998). Detection of mycoplasmas in cell cultures by cultural methods. Miles, R.J., Nicholas, R.A.J., . Methods in Molecular Biology, Vol. 104: Mycoplasma Protocols, Humana Press Inc., Totawa, NJ, 207–15, 217–26.
- Serban, M., Cotarium, D. (1970). Comparative biochemistry of MDH isozymes of vertebrates skeletal muscies. Rev. Roum. Biol. Ser. Zool. 15(3):181–187.
- O'Brien, S.J., Kleiner, G., Olson, R. . (1977). Enzyme polymorphisms as genetic signatures in human cells cultures. Science.195:1345–1348.
- Nims, R.W., Shoemaker, A.P., Bauternschub, M.A. . (1998). Sensitivity of isoenzyme analysis for the detection of interspecies cell line cross-contamination. In Vitro Cell Dev. Biol. Animal. 34: 35–39.
- Parodi, B., Aresu, O., Bini, D. . (2002). Species identification and confirmation of human and animal cell lines: a PCR-based method. BioTechniques. 32(2):432–440.
- Jenkins, N. (1999). Animal Cell Biotechnology Methods and Protocols, Humana Press Inc., Totawa, NJ, 132–138.
- Holbrook, J. J., Liljas, A., Steindel, S.J. and Rossman, M.G. (1975). Lactate dehydrogenase. Boyer, P.D. The Enzymes, Vol. XI, 3rd, Academic Press, NY, 191–292.