Abstract
To observe the histological alterations of single nerve fiber structures after nerve elongation by employing a rabbit peroneal nerve stretching model. 14 rabbits weighing mean 3. 0 kg (2.02–3.31kg.) were used in the experiment. Two rabbits were used as control when only a sham operation was done (group one, 0% stretch). Acute stretching of the peroneal nerves to elongate them by 10% was done in 6 rabbits (group two, 10% elongation) and by 20% (group three, 20% elongation) in another 6 rabbits. All animals were evaluated by tissue staining technology in a teased-fiber study. The internodal lengths were measured, and nodes of Ranvier and Schmidt-Lanterman notch were observed. The nerve fiber length was increased after stretching. The mean internodal length was 1208.31μm in group one, 1347.26μm in group two, and 1411.35μm in group three. Compared with the control group, mean internodal length was elongated by 11.50% in group two and 16.80% in group three. The difference was statistically significant. The node of Ranvier and Schmidt-Lanterman notch was wider in both group two and group three. Rupture of nerve fiber at the node of Ranvier was observed in group three. The peroneal nerve in rabbits can adapt to mild stretching by internodal length elongation. Elongation by 20% will cause structural rupture and therefore is the limit for nerve elongation.
INTRODUCTION
Brachial plexus traction injury and iatrogenic peripheral nerve injury is commonly reported. It is known that peripheral nerves can resist traction tension to a certain degree because of their histological structure and mechanical property [Citation1]. Animal experiment has confirmed that short defects of peripheral nerve can be repaired by nerve elongation. Ranvier's node, the gap in myelin facilitating action potential transmission along nerve fibers, is a specialized site that has not been studied carefully. Little is known about the change of the basic structure units of nerve fiber (Ranvier's node and internode) and its effect on nerve function during nerve elongation at present [Citation2]. In the following experiment, we stretch the peroneal nerve of a rabbit in vitro and observe the histological change of the single nerve fiber to study the effect of acute nerve elongation of varied degrees on nerve structure, aiming to find reliable experimental evidence for the application of nerve elongation in clinical practice.
MATERIALS AND METHODS
Animal and Instrument
Fourteen healthy rabbits weighing 2.5–3.0kg were provided and bred by Experiment Animal Center of People's Hospital, Peking University. Moller-Wedel Microscope for microsurgery came from Haag-Streit Inc,. Germany.
CHK Optical Microscope was from Olympus (Taiwan), 550CW Image Processing and Analysis System came from Leica (Germany), and Laser Confocal Microscopy came from Germany.
Method
Fourteen rabbits with 28 peroneal nerves were divided into 3 groups randomly. Group one had 2 rabbits as control, group two had 6 rabbits for 10% elongation of peroneal nerves, and group three had 6 rabbits for 20% elongation of peroneal nerves.
After general anesthesia by abdominal injection of 0.3% sodium pentobarbital (40mg/kg), the rabbit was fixed in pronation and bilateral peroneal nerves were exposed and anatomized. A segment of 20mm peroneal nerve was tagged by 7–0 suture stitched onto the epineurium and then severed between the tag suture. The nerve segment was fixed to a hardboard by one side and continuous traction was exerted to the other side to elongate the nerve segment to a certain length. As control, group one was not elongated (4 nerves). For group two, the 12 nerve segments were stretched to 22mm (10% elongation). For group three, the 12 nerve segments were stretched to 24mm (20% elongation).
All the nerve segments were sequentially kept in 10% formaldehyde solution for 12h, 1% osmium tetroxide for 12h, 70% glycerine for 12h, 90% glycerine for 12h, and 100% glycerine for 12h. Then the teased-fiber technique was performed to separate the single nerve fiber from the nerve segments. The single nerve fiber was anatomized from the nerve segment under Moller-Wedel Microscope and mounted onto a slide by glycerine gelatin.
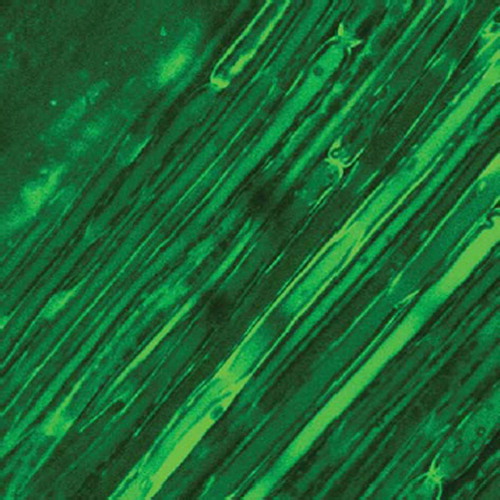
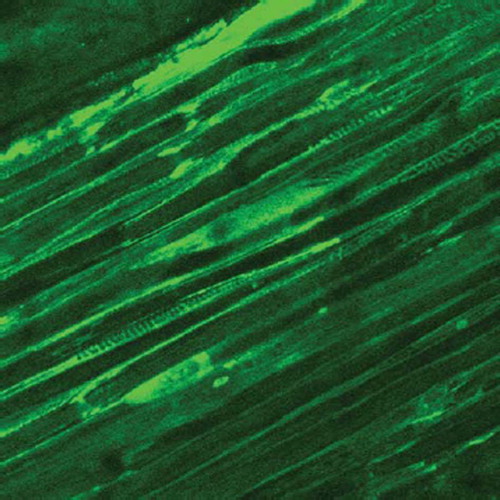
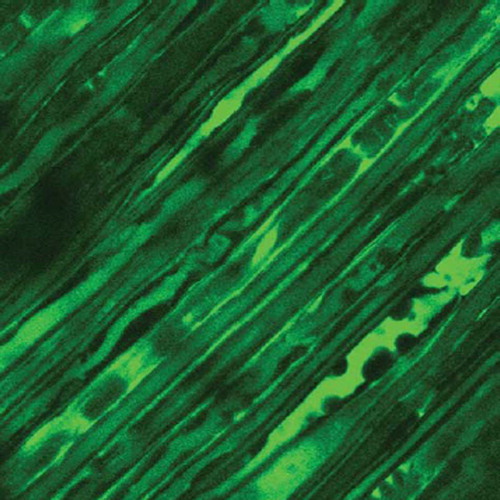
The elongated nerve segments were also carried out for Laser Confocal Detection to observe the histological changes of Ranvier's node.
Observation
Ranvier's node, internode, paranodal region and Schmidt-Lanterman notch of single nerve fiber were observed after nerve elongation under a light microscope.
The internode length of single nerve fiber was measured under a light microscope to calculate the elongation rate. For each nerve segment 10 nerve fibers were randomly picked for calculation, 3 internodes were measured for each nerve fiber, and the average length was calculated.
Data Analysis
All the data were recorded as (mean ± standard deviation) and analyzed by SPSS software (version 11.0), statistical method of t-test was adopted, P<0.05 means significant difference and P<0.01 means great significant difference.
RESULTS
Histological Change of Single Nerve Fiber after Nerve Elongation
A single nerve fiber was stained black by osmium tetroxide and the internode and adjacent paranodal sphere can be seen under a light microscope. Under a low power lens (×10), the single nerve fibers in Group 1 showed normal structure with the myelin clearly seen and the gap between the black myelin was the location of the Ranvier's node. The nerve fibers in group two were similar to those in group one with the gap of Ranvier's node clearly seen and no obvious change in its width. For group three, the gap of Ranvier's node became obviously wider than the control. Under high power lens (×40), the gap of Ranvier's node in group one measured several μm's wide and the gap of Ranvier's node in group two was several times wider than in group one. Group three had the widest gap among the 3 groups, more than 10 μm in average, and axonotmesis could be seen in the Ranvier's node in some fibers.
In a normal nerve fiber, irregularly distributed hypochromatic regions with oblique band “bamboo” shape, commonly called Schmidt-Lanterman notch, could be seen. Under high power lens (×40), the nerve fiber in group 1 had slim and narrow Schmidt-Lanterman notches. Those in group two and three were more deeply stained and wider than in group one, although no obvious difference could be observed between groups two and three.
Elongation Rate of Single Nerve Fiber
The mean internodal length was 1208. 31μm in group one, 1347. 26μm in group two, and 1411.35μm in group three. Compared with the control group, mean internodal length was elongated by 11.50% in group two and 16.80% in group three. The difference was statistically significant. The node of Ranvier and Schmidt-Lanterman notch was wider in both group two and group three. The rupture of nerve fiber at the node of Ranvier was observed in group three. The statistical t-test showed great significant difference between groups two and one (t=5.26, P<0.01), between groups three and one (t=6.67, P<0.01), and between groups three and two (t=11.08, P<0.01).
The Laser Confocal Microscopy observation of Ranvier's node can be shown in , , and .
DISCUSSION
The conduction velocity of the myelinated nerve was faster than the unmyelinated nerve because impulse jumps from one Ranvier's node to another in myelinated nerve fibers instead of spreading along the fiber in unmyelinated nerve [Citation3]. Myelin was formed by Schwann cells surrounding the axon and the Ranvier's node is the region where two Schwann cells connect, so we can say that a single myelinated nerve fiber is divided into many consecutive internodes by Ranvier's nodes and the internode is the basic structural unit of myelinated nerve fiber [Citation4]. As a specialized nervous zone the Ranvier's node has not been carefully studied until now. In this experiment, we used the teased-fiber technique to observe and measure the basic histological structure of nerve fiber, i.e. Ranvier's node, internode, and Schmidt-Lanterman notch directly and to study the effect of nerve elongation on its basic histological structure.
The peripheral nerve fiber followed a winding path just like waves, which can be stretched under external tension to adapt to the physiological limb movement [Citation5]. Because of the elastic system within the nerve fiber, the peripheral nerve can be elongated to some extent to repair its defect theoretically. Baoguo Jiang et al. [Citation6] have found that the Fontana zone may be the structure responsible for physiological elongation under external tension by stretching the sciatic nerve of rats in vitro. The existing experiments mostly focused on the study of the nervous tissue section; little was done about the change of the structure of nerve fiber after nerve elongation. In this experiment we separated the single nerve fiber by the teased-fiber technique and measured the length of internode before and after acute nerve elongation. We found an internode elongation rate of 11. 50% when the nerve was 10% elongated and an internode elongation rate of 16. 80% when the nerve was 20% elongated. There was great significant difference between the nerve elongation group and the control group and the two elongation groups were also significantly different from each other.
In our opinion, the elongation rate of the internode was less than that of the nerve because: (Citation1) the nerve fiber followed a winding path within a nerve; (Citation2) the gap of Ranvier's node and that of Schmidt-Lanterman notch became wider after nerve elongation, which may partly offset the internode elongation; (Citation3) demyelination may occur after nerve elongation, so the measurement length of internode is smaller than its actual length [Citation7].
In the physiological condition, nerve elongation can be compensated by its internal winding structure to avoid pathological change. When a nerve was elongated by 10% in our experiment, we found obvious widening of internode, Ranvier's node, and Schmidt-Lanterman notch, and tension nerve injury may have occurred. When a nerve is elongated by 20%, the internode has been stretched to its maximum length and rupture of Ranvier's node has occurred; in this case the nerve seems to be intact when seen from the outside but, in fact, great pathological damage has taken place internally.
The teased-fiber technique is the best method for studying the myelinated peripheral nerve fiber. It is useful in the observation of Schwann cell, myelin, internode, paranodal region, and axon and can help diagnose demyelination disease, early Wallerian's degradation, and secondary axon change. In pathology it is routinely used for the biopsy of fresh nerve specimens. It is a delicate procedure and tends to injure the nerve fiber; what's more, the separated single nerve fiber tends to float at the time of mounting and the dry fiber often retracts and coils, so good microsurgical technique is necessitated in the application of this technique. Based on the command of the teased-fiber technique, we clearly see the morphological change of internode, Ranvier's node, and Schmidt-Lanterman notch. Generally speaking, we recommend the use of the teased-fiber technique in the study of peripheral nerve injury. We can also observe the internode, Ranvier's node, and Schmidt-Lanterman notch clearly under the laser confocal microscopy. The laser confocal microscopy was also a new kind of technique for peripheral nerve regeneration observation.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Clark, W.L., Trumble, T.E., Swiontkowski, M.F., Tencer, A.F. (1992). Nerve tension and blood flow in a rat model of immediate and delayed repairs. J Hand Surg Am. 17(4):677–87.
- Yan, J., Jiang, B., Xu, H., Zhang, H. . (2004). The histological observation of single nerve fiber after peripheral nerve elongation. Journal of Chinese Hand Surgery. 1:58–60.
- Ramakers, G.J., Oestreicher, A.B., Wolters, P.S., van Leeuwen, F.W., De Graan, P.N., Gispen, W.H. (1991). Developmental changes in B-50 (GAP-43) in primary cultures of cerebral cortex: B-50 immunolocalization, axonal elongation rate and growth cone morphology. Int J Dev Neurosci. 9(3):215–30.
- Tomei, G., Spagnoli, D., Ducati, A., Landi, A., Villani, R., Fumagalli, G., Sala, C., Gennarelli, T. (1990). Morphology and neurophysiology of focal axonal injury experimentally induced in the guinea pig optic nerve. Acta Neuropathol. 80(5):506–13.
- Jiang, B., Zhang, P., Yan, J., Zhang, H. (2008). Dynamic observation of biomechanic properties of sciatic nerve at the suture site in rats following repairing. Artif Cells Blood Substit Immobil Biotechnol. 36(1):45–50.
- Zhang, P., Xue, F., Zhao, F., Lu, H., Zhang, H., Jiang, B. (2008). The immunohistological observation of proliferation rule of Schwann cell after sciatic nerve injury in rats. Artif Cells Blood Substit Immobil Biotechnol. 36(2):150–5.
- Jiang, B., Zhang, P., Zhang, D., Fu, Z., Yin, X., Zhang, H. (2006). Study on small gap sleeve bridging peripheral nerve injury. Artif Cells Blood Substit Immobil Biotechnol. 34(1):55–74.