Abstract
Objective: To investigate the effects of recombinant Murine RANK on the osteoclast activity. Methods: Osteoclast was observed with soluble RANK. Female Wistar rats were bilaterally ovariectomized, and intra-abdominally injected with 5 mg/Kg soluble RANK. The bone metabolism, bone density, and bone histomorphology were observed. Results: Compared with the control group, the quantity of TRAP-positive osteoclasts and bone resorption pit counting in the rest groups were significantly reduced. The bone density of the dosed groups was significantly increased and TRAP-stained osteoclasts in bone tissue sections were almost inhibited. Conclusion: rh-Murine RANK was able to inhibit osteoclast differentiation and prevent ovariectomy-induced bone loss.
The OPG/RANKL/RANK system is an important signal transduction pathway in the process of osteoclast differentiation. It includes ligand RANKL (ligand of receptor activator of NF-κB) [Citation2], receptor RANK (receptor activator of NF-κB) [Citation3], and the pseudo-receptor of RANKL - osteoprotegerin (OPG) [Citation1].
RANK is the sole target receptor in RANKL stimulating the secretion and mature of osteoclasts [Citation9,Citation15]. RANKL activates the receptor RANK in the cell membranes of osteoclast precursors, promoting the proliferation, differentiation, maturation, and bone resorption activity of osteoclasts [Citation3]. OPG, as a decoy receptor of RANKL [Citation9], primarily through binding with RANKL, competitively inhibits the combination of RANKL with the receptor RANK in the cell membrane of osteoclast precursors, and eliminates the effects of RANKL on osteoclasts, thereby inhibiting bone resorption and increasing bone mass.
However, studies found that OPG can also competitively inhibit tumor necrosis factor-related apoptosis-inducing factor (TRAIL)-mediated apoptosis [Citation11,Citation12,Citation13,Citation14], and on this mechanism OPG plays the role of tumor cell-activating factors [Citation12,Citation14]. Therefore, the use of OPG in treating osteolytic damage arising from malignant tumors metastasia is restricted. J.G. Emery et al. [Citation13] also believed that although the physiological response in vivo resulted from the binding of OPG and TRAIL is unclear, it is certain that the combination of OPG with TRAIL will weaken its role of inhibiting osteoclast activity to some extent.
Recent studies have found that, in addition to the form of transmembrane protein, RANK also exists in the circulatory blood in the form of soluble protein. It can combine with RANKL, but is not able to mediate the continued differentiation of osteoclast precursors, nor promote the aggregation and migration of osteoclast precursors, showing a role of inhibiting osteoclasts similar to OPG [Citation10]. The soluble recombinant RANK-FC protein combined with human immunoglobulin IgG-Fc fragment can inhibit the RANK-regulated osteoclast differentiation and activity [Citation2,Citation6,Citation9,Citation10] while in some in vivo experiments [Citation4, Citation8, Citation10]. Besides, RANK was the only ligand of RANKL that induced the downstream signaling pathway [Citation10,Citation15], and RANK only bound with RANKL [Citation2], not with TRAIL [Citation14]. Therefore, in the treatment of osteolytic damage caused by malignant tumors, it is more beneficial for RANK to combine with RANKL and not with TRAIL.
Many scholars have made useful investigations based on the effect of the RANK in inhibiting osteoclastic bone resorption [Citation5, Citation7, Citation14]. However, there is no report on the prevention and treatment of primary osteoporosis such as postmenopausal osteoporosis. Since RANK was discovered, there is yet no report on the expression and purification of RANK protein in E. coli. We applied the soluble RANK protein (amino acid 26-210) expressed in a prokaryotic expression system (E. coli strain BL21-Gold) for the first time, and observed its effects on osteoclast differentiation and resorption in both in vivo and in vitro experiments, as well as its role on the prevention of ovariectomy-induced bone loss. Its mechanism of action on the prevention and treatment of metabolic bone disease was also investigated to provide a theoretical basis for its clinical use in future.
MATERIALS AND METHODS
Materials and Equipment
In this experiment, 10 SD fetal rats with the age of <24h were provided by the Laboratory Animal Center, General Hospital of the People’s Liberation Army (license number: SCXK-(Jun) 2007–004); 26 clean female Wistar rats, 3 months old, weighing 250–270g, were provided by the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences (license number: SCXK (Beijing) 2004–0001); Hanks buffer, DMEM medium (Sigma), fetal bovine serum (Gibco), TRAP staining kit 387A (Sigma) and 10% chloral hydrate solution were provided by the Department of Pharmacy, General Hospital of the People’s Liberation Army.
Recombinant Murine RANK protein (Met26-Pro210) was donated by the Institute of Microbiology, Chinese Academy of Sciences. The recombinant plasmid DNA encoded the extracellular domain of RANK receptor in RAW264.7 cell (Met26-Pro210), and was expressed in E. coli BL21. The expressed RANK protein mainly existed in the form of inclusion bodies, and after separation, purification, renaturation, detection by FPLC and analysis on Superdex75, Superdex200 gel filtration columns, the renatured RANK protein existed as monomers in solution with a molecular weight of 26KD.
Preparation of thin bone sections: with the use of a hacksaw and fine and coarse emery stones, fresh bovine femoral cortical bones were prepared into 6 mm × 6 mm and 30 μm thick ground bone sections. The sections were washed in a ultrasonic cleaning machine with distilled water 3 times, each time for 10 min. After being sterilized with Co-60 radiation, they were placed in 6-well plates pending use.
Instruments: CO2 incubator for in vitro cell culture (Heraeus, Germany), and inverted phase contrast microscope (Olympus IX70, Japan), Beckman high-speed refrigerated centrifuge (Beckman Instruments Inc, USA), critical point drying instrument (Hitachi HCP-2), vacuum ion beam sputtering instrument (Hitachi E-102), scanning electron microscope (Hitachi S-4800 ultra-high-resolution scanning electron microscope), Olympus 5400 digital camera (Olympus Corporation), Leitz slicer (Leitz Inc.), Norland XR-36 densitometer (Norland Corporation, U.S.), drying machine (Tianjin Tianli Aviation Electro-Mechanical Co., Ltd., KPJ-1), cell spreading machine (Tianjin Tianli Aviation Electro-Mechanical Co., Ltd., ZPJ-1), electronic analytical balance (Shanghai No. 2 Balance Machinery Co. Ltd., MA260S).
Osteoclasts Culture
Mechanical Separation and Osteoclasts Primary Culture. The newborn SD fetal rats, after being rinsed in distilled water, were killed by cramping in the neck, and immersed in 75% alcohol for 2 min. The limbs were placed in cold Hanks buffer, and we isolated the tibia, femur, humerus, ulna and radius with ophthalmic scissors and tweezers, removing the soft tissue around the bone as much as possible. Then the bones were placed in cold DMEM media, with 0.5–1 ml DMEM for each rat. In the separating medium, with the use of a scalpel the substantia ossea was rapidly scratched to pieces, removed into 10 ml centrifuge tubes, repeatedly blown and beaten with a round dropper for 2 min until the osteoclasts attached on the bone matrix for 15–30 s. The upper cell suspension was drawn, diluted with DMEM culture medium to 1 × 105/ml, inoculated into a coverslip in six-well plates or ground bone systems, and cultured in 37°C 5% CO2 incubators. Then it was observed for cell growth under an inverted phase contrast microscope.
Observation, Staining, Identification, and Counting of Osteoclasts. The morphology of osteoclasts in the six-well plates was observed under an inverted phase contrast microscope. After about six days, when the osteoclasts were able to be obviously observed, TRAP staining, identification and counting were performed. TRAP staining: the cells in the coverslips were fixed with citric acid/acetone for 30 s, incubated in TRAP staining and incubation solution at 37°C for 50 min, and after being washed with distilled water were mounted with glycerogelatin. The multinucleated giant cells with more than two positive-stained nuclei observed under light microscope were osteoclasts. Ten visual fields under a ×100 light microscope were selected and the average number of osteoclasts was recorded.
The coverslips in six-well plates were divided into 4 groups: the control group without Murine RANK protein, and three experimental groups with RANK protein concentrations of 10–4 mol/L, 10–5 mol/L, 10–6mol/L, respectively, and the same other culture conditions. The solution was changed and dosed every other day. Three days after dosing, TRAP staining was performed, 10 visual fields under a ×100 light microscope were selected, and the average number of osteoclasts was recorded.
Observation of the resorption pit in ground bone sections: the bone sections were divided into four groups as the above-mentioned method. About 6 days after cell culture, the sections were added with various concentrations of the solution at the same time with the coverslip groups and cultured under the same conditions. The solution was changed and dosed every other day. Three days after dosing, the bone sections in each group were taken out, fixed with 2.5% glutaraldehyde, stained with toluidine blue, and the positively stained lacunae in the entire bone section were counted under a ×100 light microscope. The stained bone sections were washed in distilled water by ultrasonic cleaning for 5 times, each time for 5 min. Then scanning electron microscope specimens were prepared by the conventional method, observed under scanning electron microscope, and the resorption lacunae were photographed.
Animal Experimental
In this study, all animal experiments were made under the guidance of the medical ethics committee of the General Hospital of People’s Liberation Army, which was in compliance with the requirements of the ethics of animal experiments.
Grouping. Twenty-six 3-month-old female Wistar rats were weighed. According to body weight, the rats were randomly divided into 3 groups: the sham-operated group (the sham group) (n=6), the ovariectomized RANK group (the RANK group) (n=9), the ovariectomized placebo group (the OVX group) (n=11). Ovariectomized surgery on female rats: rats were intraperitoneally injected with 0.3 ml/100g body weight of 10% chloral hydrate solution; after 10 min when satisfactory anesthetic effect was achieved, the rats were fixed at a prone position. An incision was made at the middle of the back, the subcutaneous tissue was separated to the sides, the fascia lumbodorsalis and underlying muscle were incised, and that side of the abdominal cavity was squeezed with fingers so that the retroperitoneal fat was revealed from the incision. At about 1 cm below the kidney, a pink uterine (2–3 mm in diameter) and a pink mulberry-shaped ovarian were seen. After the uterine tube was ligated, the ovary was separated and resected, and then the fascia was sutured. The other ovary was resected in the same way 80,000 u of penicillin was administered into the incision, and then the skin was sutured. For the Sham group, the uterine tubes were ligated and the ovaries were separated, while no ovariectomy was performed. After operation, conventional dressing was made. During the three days postoperatively, 80,000 u of penicillin was delivered daily to prevent infection, and seven days after operation the suture was removed. All the animals were reared under the same conditions and with the standard feed (containing 1.66% calcium, phosphorus 1.24%).
Drug Delivery. One week after operation, the suture was removed and rats were administered with the drug. The RANK treatment group was intra-abdominally delivered with 5mg/kg body weight of RANK protein three times a week for 12 consecutive weeks. The Sham group and the ovariectomized control group were delivered with 0.2ml PBS buffer in the same manner.
Evaluation of Bone Loss
Weekly Body Weight Measurement. Preoperatively and postoperatively, body weights were measured every week. Body weights were compared between groups.
Detection of Serum Calcium, Phosphorus, Alkaline Phosphatase, and Osteocalcin. Twelve weeks after administration, rats were intraperitoneally injected with 0.3 ml/100g body weight of 10% chloral hydrate solution for anesthesia. After 10 min, when the satisfactory anesthetic effect was achieved, 1 ml of blood was drawn from the back of the eye and centrifuged at 1500 rpm at room temperature for 10 min. The serum samples were used for serum calcium, phosphorus, alkaline phosphatase, and osteocalcin concentrations measurement.
Bone Density Testing. Twelve weeks after administration, after being anesthetized with 10% chloral hydrate, rats were put under the Norland XR-36 bone density instrument and scanned for bone density of the whole body using a small animal model. BMD (g/cm2) of the whole body was firstly automatically measured, and then the femur, tibia, and lumbar spine BMD.
Bone Morphology Parameters. Histopathological detection: the left femur and tibia of five rats in each group were fixed in 10% formaldehyde for 3 days, and decalcified pathological sections were routinely prepared. Routine HE staining and TRAP staining were made to observe the trabecular structure and osteoclast inhibition status.
Statistic
All data were analyzed with the SPSS13.0 statistical analysis software. The measurement indicators were expressed in mean ± standard deviation (x ± s). The analysis of variance was used in multi-group comparison, and t tests were performed for comparison between the two groups. P < 0.05 was considered to be statistically significant.
RESULTS
In Vitro Experiments
Two hours after inoculation, most of the osteoclasts obtained by mechanical separation were adhered to the wall, and the cells were generally small and round. After about 2 h, the cells were partially unfolded, and some cells were in a large area, with polynucleation and irregular shape (), and each type of cells was growing well. Six days after culture, the slides of the control group began to show a varying number of multinucleated giant cells, and the cell morphology under a microscope was clear, in fried-egg-like, long strip, funnel-shaped or irregular shapes, with patchy or filopodia. These cells were rich in cytoplasm, with several to a dozen nuclear, and cytoplasmic vacuoles of varying sizes were able to be seen. The cell shapes were changing with longer culture time. Tartrate-resistant acid phosphatase staining (TRAP) were made, to detect mature osteoclasts. TRAP staining showed that the multinucleated cytoplasm was in rose pink (), which were demonstrated to be mature osteoclasts. Then the drug was delivered in each group, and three days after administration, TRAP-positive cells in the 10–4 mol /L group, the 10–5 mol/L group, and the 10–6mol/L group decreased significantly. TRAP-positive cells in the control group were far more than in the experimental group, and with the increase in the concentrations of RANK, TRAP-positive cells were in a decremental trend. Intergroup comparison showed that the number of TRAP-positive cells in the 3 experimental groups were significantly less than the control group (P < 0.01), indicating that RANK inhibited osteoclasts in vitro ().
Figure 1. (×300): Four hours after inoculation, most of the osteoclasts obtained by mechanical separation were adhered to the wall. The cells were generally small and round, and partially spread. Some cells were large in area, polynuclear, and in irregular shapes.
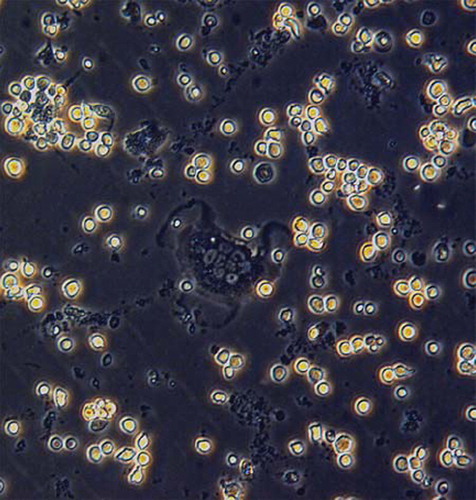
Figure 2. (×300): TRAP staining and identify was performed about six days after culture. TRAP staining showed that the cytoplasm of polykaryocytes was rose pink, which was demonstrated to be mature osteoclasts.
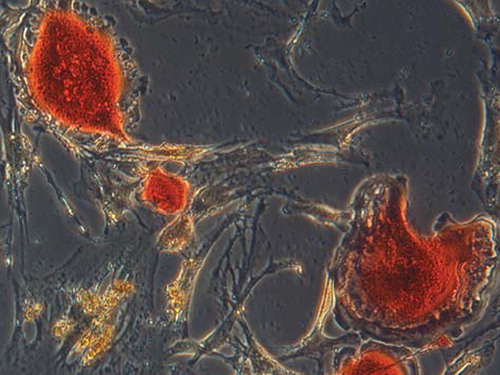
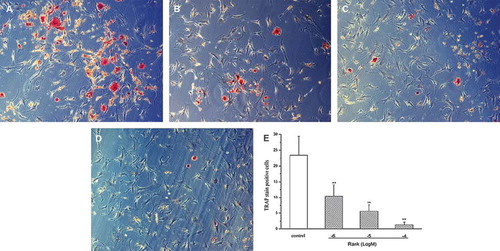
Figure 3. (×150): Three days after drug delivery. The control group showed plenty of TRAP staining-positive osteoclasts. Compared with the control group, the RANK groups decreased significantly. A: the control group; B: 10−6 mol/L group; C: 10−5 mol/L group; D: 10−4 mol/L group; E: for the TRAP staining-positive cells counting (n = 10), the number of TRAP-positive cells in the RANK groups significantly reduced compared with the control group, which was in a dose-dependent manner (×100) and was statistically significant (P <0.01).
The morphology of osteoclasts observed under a scanning electron microscope weas in diversified shapes, which was identical with that under an inverted phase contrast microscope, and large and flat tulle-like pseudopodias were visible, cell surface with a large number of protrusions (). Resorption pits were round, oval, rough on the bottom and with a clear border (). Three days after administration, the number of bone resorption pits and different concentrations of RANK protein significantly inhibited osteoclastic bone resorption on ground bone sections ().
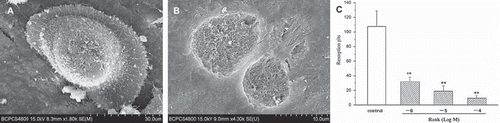
Figure 4. Scanning electron microscopy. A: The morphology of osteoclasts in the bone sections were clear, in fried-egg-shaped long strips; funnel or irregular shapes and large and flat tulle-like pseudopodias could be seen, with a large number of filamentous processes in the cell surface. B: The bone resorption pits were round or oval, which were rough on the bottom and clear in border. C: Bone resorption pits counting of bone sections (n = 10) by toluidine blue staining; under a ×100 inverted phase contrast microscope, the staining-positive pits in an entire bone section were counted. We found that, compared with the control group, the toluidine blue staining-positive resorption pit counting of the RANK groups decreased significantly, with a statistically significant difference (P<0.01).
In Vivo Experiments
Post-operatively, all the animals had a good wound healing. After administered with drugs, the animals in the experimental groups were observed with normal diet and activities.
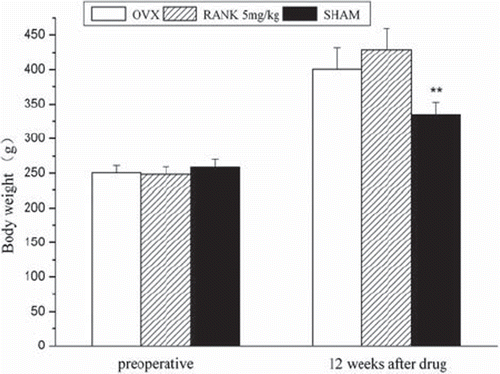
Results of Body Weight Measurements. There was no significant difference in the pre-operative body weight of rats in each group. Twelve weeks after delivery, the body weight significantly increased in each group. Analysis of variance performed with a Spss 13.0 statistical software showed that pre-operatively there was no difference between the groups (P > 0.05). Twelve weeks after delivery, the sham group had the lowest weight (P < 0.05), and there were significant differences among the groups ().
Figure 5. Preoperatively, F = 1.506, P > 0.05; 12 week after administration, F = 20.408, P< 0.05; preoperatively and postoperatively body weights were measured every week, and preoperatively there was no significant difference in body weights between each group, F = 1.506, P > 0.05; 12 weeks after delivery there was a significant difference in body weights of rats between the OVX groups and the sham group (P = 0.00029), but no significant difference between the OVX group and the RANK group (P = 0.05254).
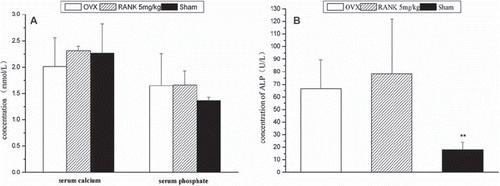
Test Results of Serum Calcium, Phosphorus, Alkaline Phosphatase, and Osteocalcin. When analyzed with the Spss 13.0 statistical software, there was no significant difference in the serum calcium values between the groups (P > 0.05). There were significant differences in the serum phosphorus values between the groups, with the highest in the OVX group, second in the RANK group, and the lowest in the sham group. For the intergoup comparison on the ALP values, there was significant difference between the sham group and each of the rest of the groups. A comparison between the OVX group and the RANK group showed P > 0.05, while values in the RANK group were significantly higher ().
Figure 6. Twelve weeks after drug delivery, rat serum biochemical detection. (A): There was no significant difference in serum calcium values between the groups (P > 0.05); phosphorus values in each group were significantly different, with the highest in the OVX group, second in the RANK group, and the lowest in the sham group. (B): In group comparisons on the ALP values, the sham group was significantly different from the rest groups; a comparison between the OVX group and the RANK group showed P > 0.05, but the values in the RANK group were significantly higher (calcium: F = 1.186, P > 0.05; phosphate: F = 1.004, P > 0.05; alkaline phosphatase: F = 8.03, P<0.05.
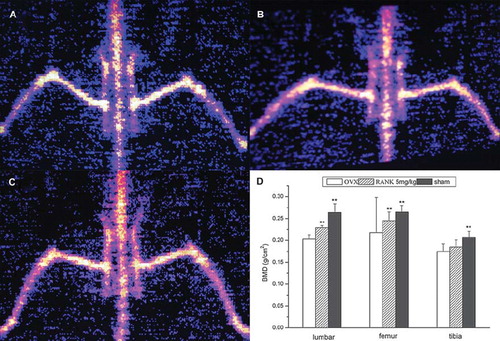
Bone Density. Spss 13.0 statistical analysis of the bone density of the lumbar spine showed differences between the groups, with the highest in the bone density of the sham group (P = 0.00000) and second in the RANK group (P = 0.00000). Compared with the OVX group, there was significant difference in each group. Femur bone density was highest in the sham group (P=0.00000), and the RANK group (P = 0.00051) was higher than the OVX group, with differences among the groups. For tibial bone density, there were differences between the sham group and each of the rest of the groups; intergroup comparison showed that there was no obvious difference between the OVX group and the RANK group (P = 0.25897), but bone density of the RANK group was slightly higher than the OVX group ().
Figure 7. Bone density detection showed that 12 weeks after ovariectomy the bone density of the lumbar spine and femur in the OVX group (A) decreased significantly, and there was a significant difference compared with the sham group (C), indicating that 12 weeks after ovariectomy, the bone osteoporosis models were established; the bone density in the RANK group (B) was significantly higher than the OVX group, indicating that RANK significantly inhibited the ovariectomy-induced bone loss, while the tibial bone density of the OVX group was not statistically different from the RANK group (D) (lumbar spine: F = 54.867, P < 0.000; femur: F = 21.69, P <0.000, tibia: F = 7.299, P < 0.01.
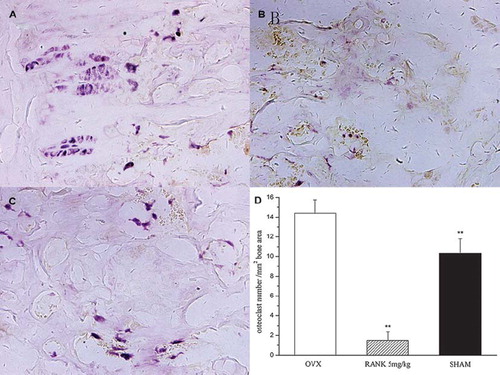
Detection of Bone Histomorphology. Observation under the microscope for bone trabecula in distal 2 cm and proximal 2 cm of proximal femoral epiphyseal plate of distal femoral epiphyseal plate: the sham group was longitudinally arranged and interlaced into a mesh-like pattern. When compared with the sham group, the trabecula of the OVX group became smaller, more sparse, significantly reduced, the number of blind-side increased, and lossed the normal reticular structure. When compared with the OVX group, the trabecula of the RANK group became thickened, arranged closely, and basically remained in normal reticular structure (). TRAP staining of distal femur sections showed that in the RANK group osteoclasts was almost inhibited, while the OVX group and the sham group had more osteoclasts. Observed under ×100 microscope, TRAP staining positive-cell counting showed that the RANK group was the lowest, and there was a significant difference with the remaining group, P < 0.05 ().
Figure 8. Observation of trabecular structure by HE staining showed that the trabecular structure in the sham group was longitudinally arranged and interlaced into a mesh-like pattern (C); compared with the sham group the trabeculae in the OVX group (A) were thinner, more sparse, the number was significantly reduced and blind sides increased, and the normal reticular structure disappeared. Compared with the OVX group, the trabeculae of the RANK group (B) were thicker, more closely arranged, and substantially maintained the normal reticular structure.
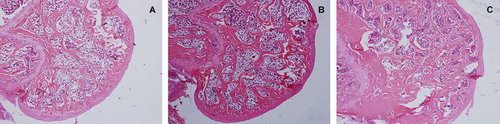
Figure 9. TRAP staining of decalcified sections of the distal femur showed that osteoclasts of the RANK group (B) were almost inhibited, while in the OVX group (A) and the sham group (C) there were many TRAP staining-positive osteoclasts. (D): Osteoclast counting in distal femur by TRAP staining; under a ×100 endoscope TRAP staining-positive cells of the distal femur sections were counted and we found that the lowest was the RANK group, and there were significant differences with the rest of the groups (F = 264.588, P< 0.01).
DISCUSSION
The maintenance of normal bone morphology depends on the dynamic balance between bone formation and bone resorption, in which osteoclast regulation is particularly important for the maintenance of this balance. Osteoclasts are multinucleated cells derived from the fusion of the monocyte precursor cells of monocyte/macrophage lines. In the process of bone growth and development, it can continuously resorp bone matrix, and complete bone remodeling with the synergy of osteoblasts, and its formation and resorption function are affected by many factors. Research in recent years [Citation5] found that many hormones and cytokines, such as 1, 25 (OH) 2D3, parathyroid hormone (PTH), prostaglandin (PGE2), and interleukin (IL-1, IL-6), directly or indirectly regulate OPG, RANKL expression and the ratio of OPG and RANLKL, thereby mediating generation of osteoclasts (OC) and maintaining its functions to achieve the action of anti-osteoporosis or osteoporosis.
This study first observed the inhibition status of recombinant RANK protein on osteoclasts in experiments in vitro. Because so far there is no cell strain with the characteristics of a mature osteoclast, it is a major channel of obtaining osteoclasts by isolating them from the bone tissues or bone marrow-indution. In this study, through direct isolating and culturing osteoclasts from neonatal rats, the yielded osteoclast morphology was typical, with a long survival time and active function, although the culture was mixed with marrow stromal cells and other cells. It was observed in the experiments that 3 d after the action of RANK protein, part of the osteoclasts became round, and the nucleus disappeared. There were statistically significant differences in the number of TRAP-positive cells between the RANK group and the control group. Counting of ground bone resorption pits was significantly reduced, and a scanning electron microscope showed that the area and the depth of bone resorption pits also decreased significantly. Also in this experiment, after about six days when osteoclasts were fully mature, TRAP staining and identification were performed. The inhibition status after administration was observed, and 3 d after administration identification was made, as we considered that the survival of osteoclasts was about 9-13d. At its end stage of growth, its own activity would decline, and TRAP-positive cells would be sure to reduce. This natural decline of osteoclasts may cover up the role of the RANK protein to some extent. Therefore, we observed another experiment. In the experiment, about 4 h after the cells inoculated to slides, after the majority of cells were observed to be adhered to the wall, RANK protein was added as the aforementioned method and grouping, with the same culture method. After about 6 days, osteoclast activity was observed under an inverted phase contrast microscope and by TRAP staining, and found that compared with the control group the number of TRAP staining-positive osteoclasts of the different concentrations was significantly reduced, and in a dose-dependent manner. This proves that recombinant RANK protein in this experiment has strong inhibitory effects on osteoclasts.
In the in vivo experiments, we established a model with ovariectomized female Wistar rats. One week after ovariectomy, the prevention group were intraperitoneally delivered with 5mg/kg body weight of RANK protein three times a week for 12 weeks and a sham control group and a blank control group were set to observe the preventive effect. Twelve weeks after being ovariectomized, it was found that the body weight of rats in all the groups increased significantly and was most significant in the ovariectomized rats, with significant difference compared with the sham group, which was consistent with the compensatory weight gain after estrogen deficiency and also in line with literature reports [Citation16]. Twelve weeks after delivery, serum calcium values were found to have no significant difference between the groups (P > 0.05). There was difference in phosphorus values between the groups, with the highest in the OVX group, second in the RANK group, and the lowest in the sham group. ALP is a commonly used indicator in evaluation of bone turnover and bone formation. ALP values in the OVX were significantly higher than the sham group, indicating that post ovariectomy bone turnover significantly accelerated, demonstrating that the osteoporosis model proved to be successful. But there were no significant differences in the ALP values between the OVX group and the RANK group (P > 0.05)."Bone density detection showed that 12 weeks after ovariectomy the bone density of the lumbar spine and femur in the OVX group decreased significantly, and there was a significant difference with the remaining group, indicating that 12 weeks after ovariectomy osteoporosis models were successfully established. The bone density in the RANK group was significantly higher than the OVX group, indicating that RANK significantly inhibited ovariectomized-induced bone loss. While compared with the sham group the bone density in the RANK group was slightly lower, the difference was not statistically significant, which also suggested that a simple inhibition of osteoclasts could not completely recover to normal bone mass. TRAP staining of distal femur sections showed that osteoclasts in the RANK group were almost inhibited, while there were more osteoclasts in the OVX group and the sham group, with significant differences compared with the RANK group. This demonstrates that in the in vivo experiments recombinant RANK protein had strong inhibitory effects on osteoclasts and on ovariectomy-induced bone loss, but could not completely recover to normal bone mass.
CONCLUSION
In summary, our study demonstrates that recombinant murine RANK protein is an effective antagonist of RANKL; in in vitro experiments it can effectively inhibit osteoclast differentiation and its bone resorption activity; in in vivo experiments it can effectively inhibit osteoclast cell activity and prevent ovariectomy-induced bone loss, which brings new hope for the prevention and treatment of osteoporosis.
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Simonet, W.S., Lacey, D.L., Dunstan, C.R., . (1997). Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89: 309–319.
- Anderson, M.A., Maraskovasky, E., Billingsley, W.L., . (1997). A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390: 175–179.
- Udagawa, N., Takahashi, N., Matsuzaki, K., . (1998). Osteoblasts/stromal cells activate osteoclase function through osteoclast different ion factor (ODF). Second Joint Meeting of the ASBMR and the IBMS, San Francisco, CA, 1998.
- Miller, Robert E., Branstetter, Daniel, Armstrong, Allison, . (2007). Receptor activator of NF-B ligand inhibition suppresses bone resorption and hypercalcemia but does not affect host immune responses to influenza infection. The Journal of Immunology 179: 266–274.
- Sordillo, E.M., Pearse, R.N. RANK-Fc: A therapeutic antagonist for RANK-L in myeloma. American Cancer Society 97 (3): 802–812.
- Wang, Bao-Li, Liang, Hui, Zheng, Fang, Li, Xiao-Xia, . (2007). Recombinant soluble receptor activator of nuclear factor-κB inhibits parathyroid hormone-induced osteoclastogenesis in vitro. Acta Physiologica Sinica 59 2: 169–174.
- Feeley, B.T., Liu, N.Q., Conduah, A.H., . (2007). Mixed metastatic lung cancer lesions in bone are inhibited by noggin overexpression and rank: Fc administration. J Bone Miner Res. 22 (3): 486.
- Hsu, H., Lacey D. L., Dunstan, C. R., Solovyev, I., Colombero, A., Timms, E., Tan, H-L., Elliot, G., Kelley, M. J., Sarosi, I., Wang, L., Xia, X-Z., Elliot, R., Chiu, L., Black, T., Scully, S., Capparelli, C., Morony, S., Shimamoto, G., Bass, M. B., Boyle, W. J. (1999). Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc. Natl. Acad. Sci. USA 96: 3540–3545.
- Nakagawa, N., Kinosaki, M., Yamaguchi, K., . (1998). RANK Is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochemical and Biophysical Research Communications 253, 395–400.
- Babatunde, O., Anderson, D.M., Traianedes, K. . (2001). Therapeutic efficacy of a soluble receptor activator of nuclear factor kB-IgG Fc fusion protein in suppressing bone resorption and hypercalcemia in a model of humoral hypercalcemia of malignancy. Cancer Research 61: 2572–2578.
- Shipman, C.M., Croucher, P.I., . (2003). Osteoprotegerin is a soluble decoy receptor for tumor necrosis factor-related apoptosis-inducing ligand/Apo2 ligand and can function as a paracrine survival factor for human myeloma cells. Cancer Res 63: 912–916.
- Holen, I., Croucher, P.I., Hamdy, F.C., Eaton, C.L., . (2002). Osteoprotegerin (OPG) is a survival factor for human prostate cancer cells. Cancer Res 62: 1619–1623.
- Emery, J. G., McDonnell, P., Burke, M. B., Deen, K. C., Lyn, S., Siverman, C., Dul, E., Appelbaum, E. R., Eichman, C., Diprinzo, R., Dodds, R. A., James, I. E., Rosenberg, M., Lee, J. C., Young, P. R. (1998). Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J. Biol. Chem 273: 14363–14367.
- Zhang, J., Dai, J., Yao, Z., Lu, Y., Dougall, W., Keller, E.T. (2003). Soluble receptor activator of nuclear factor kappaB Fc diminishes prostate cancer progression in bone. Cancer Res 63: 7883–7890.
- Li, J., Sarosi, I., Yan, X-Q., Morony, S., Capparelli, C., Tan, H-L., McCabe, S., Elliott, R., Scully, S., Van, G., Kaufman, S., Juan, S-C., Sun, Y., Tarpley, J., Martin, L., Christensen, K., McCabe, J., Kostenuik, P., Hsu, H., Fletcher, F., Dunstan, C. R., Lacey, D. L., Boyle, W. J. (2000). RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc. Natl. Acad. Sci. USA 97: 1566–1571.
- Bagi, C.M., Ammann, P., Rizzoli, R., . (1997). Effect of estrogen deficiency on cancellous and cortical bone structure and strength of the femoral neck in rats. Calcif Tissue Int. 61: 336–344.
- Cheng, X., Kinosaki, M., Takami, M., . (2004). Disabling of receptor activator of nuclear factor-kappaB (RANK) receptor complex by novel osteoprotegerin-like peptidomimetics restores bone loss in vivo[J]. J Biol Chem 279 (9): 8269–8277.