Abstract
Abstract: Viscosity of blood substitutes is among the important determinants to restore microcirculation. Sodium alginate (SA) is always mentioned as “viscosity modifier” in creating blood substitutes. In the present study, the whole blood was diluted using SA solutions to final hematocrits of 10%, 20%, and 35%, respectively. The whole blood viscosity (WBV) at different shear rates, plasma viscosity (PV), and rheological behavior of red blood cells (RBCs) was studied in vitro. The results show that SA may induce RBCs aggregation in a dose-dependent manner. Furthermore, the effect of SA on RBCs aggregation maybe involve the regulation of microcirculation.
INTRODUCTION
Plasma expanders, namely plasma substitutes, are widely used in hemodilution and the treatment of hemorrhagic shock. Viscosity of plasma expanders usually used is similar to or lower than the normal plasma viscosity of ∼ 1.2cp. It was generally perceived that the lowered blood viscosity in hemodilution and hemorrhagic shock could improve the tissue perfusion due to reduced flow resistance. But the study of Tsai AG et al. showed that increased plasma viscosity was beneficial in extreme hemodilution and hemorrhagic shock [Citation1]. Cabrales P et al. found that extreme hemodilution performed with HVPE yielded systemic arterial pressures and functional capillary densities that were significantly higher than those obtained with 6% Dextran 70, a widely used plasma expander [Citation2]. The use of HVPE in extreme hemodilution maintained the number of perfused capillaries and tissue perfusion by comparison with a low viscosity plasma expander (LVPE), due to increased mean arterial blood pressure and capillary pressure [Citation3]. During acute extreme anemic conditions, increased mean arterial pressure, cardiac output, and shear stress could be the possible mechanisms of maintaining organ and microvascular perfusion after exchange with HVPE compared to LVPE [Citation4]. In addition, their work suggested the possibility of using HVPE to prolong the period for initial treatment of blood losses, because the HVPE showed improved and longer-lasting effects relative to the conventional low viscosity [Citation5]. HVPE resuscitation also provided a more consistent and prolonged resuscitation than traditional hyperoncotic treatment in hemorrhagic shock [Citation6], because high plasma viscosity was beneficial for maintaining microvascular recovery compared with low plasma viscosity [Citation7].
Plasma expanders or blood substitutes with low viscosity may decrease blood viscosity and then the systemic vascular resistance. However, events at the level of microcirculation may not necessarily reflect systemic hemodynamics. A drop in viscosity causes a lower shear stress, which triggers down-regulation of nitric oxide (NO) by endothelial cells and leads to vasoconstriction [Citation8]. Elevated plasma viscosity in extreme hemodilution can increase perivascular NO concentration and microvascular perfusion [Citation9]. Besides, the resuming of blood viscosity improves hemorrhagic shock resuscitation independently of the restitution of oxygen-carrying capacity [Citation10,Citation11]. Thus, viscosity of plasma expanders or blood substitutes is among the important determinants of ability to restore microcirculation.
SA is widely used in tissue engineering, dental material, and drug microencapsulation. SA of a comparatively low concentration can result in high viscosity with a low COP, thus it was used as viscogenic materials in the study on the effects of HVPE [Citation2,Citation5,Citation6]. Silva et al. used SA as “viscosity modifier” in perfluorocarbon emulsion (A.C. Silva, unpublished observation). During the middle of the last century, SA of lowered polymerization has been used as a plasma expander in Japan [Citation12,Citation13], but was disused due to its renal toxicity [Citation14]. Now most researchers choose the SA of higher polymerization to greatly eliminate the negative effects on the kidney. For example, Cabrales P et al. used a type of SA with the molecular weight of 75∼200KD [Citation2].
Although SA was used as the plasma expander to elevate plasma viscosity in many studies, its effects on WBV and rheological behaviors of RBC were poorly understood. In the present study, we expect to provide some evidence that SA may affect WBV and the RBC rheological behaviors. The SA was an ultrapure product that was free of endotoxins. WBV, PV, RBC deformability and aggregation were investigated.
MATERIALS AND METHODS
Tested Solutions
High viscosity solutions were prepared using 0.6% and 0.3% of PRONOVA UP LVM alginate (FMC biopolymer, Sandvika, Norway) in 0.9% NaCl. Manuronic acid content of LVM alginate powder was 54%. The control solution was 6% D70 (70kDa MW, Pharmacia Corporation, Uppsala, Sweden) in 0.9% NaCl. All solutions were filtered by 0.22μm Acrodisc Syringe Filter. The tested solutions were labeled A0.6% (0.6% LVM alginate in 0.9% NaCl), A0.3% (0.3% LVM alginate in 0.9% NaCl) and D6% (6% D70 in 0.9% NaCl).
Viscosity of the solutions was tested by Rotational Rheometer (CV100, HAAKE, Germany) and expressed in mPa.s. COP was measured using a membrane colloid osmometer (model 4420, Wescor, Logan, UT). lists the viscosity and COP of the three tested solutions.
Table 1. Viscosity and COP of the solutions
Blood Samples and Experimental Protocols
Blood sampling. Blood samples were obtained from healthy adult volunteers and informed consent was obtained. Heparin treated tubes were used to collect blood. Blood was centrifuged at 1500 × g for 10 min at 4°C. Plasma was removed and the concentrated RBCs were subsequently mixed with tested solutions as appropriate.
Viscosity. The whole blood was diluted in A0.6% and A0.3% solutions to final hematocrits of 10% (10% RBC + 10% Plasma + 80% tested solution), 20% (20% RBC + 20% Plasma + 60% tested solution) and 35% (35% RBC + 35% Plasma + 30% tested solution), respectively. The WBV at different hematocrit and PV at final hematocrit of 35% were measured at 37°C immediately after mixing.
RBC deformability and aggregation. The whole plasma volume was replaced with tested solutions (30%) and the plasma (35%) to a final hematocrit of 35%. RBC deformability and aggregation were measured after blood samples were incubated in replacing solutions for 30 min at 37°C.
Measurements of Hematocrit, WBV, and PV
The hematocrit of blood samples was determined by semi-automated Microcell Counter F-820 (Sysmex Corporation, Kobe, Japan). WBV was determined at shear rates of 10 s−1, 60 s−1 and 150 s−1 by using a cone-plate viscometer (Model LBY-N6B, Precil Company, Beijing, China). WBV is influenced by hematocrit, RBC deformability, aggregation, and plasma viscosity. At low shear rates, blood viscosity values are indicative of RBC aggregation, and at higher shear rates RBC deformability has a prominent influence. Thus, blood viscosity levels between 50 s−1 and 150 s−1 are indicative of an abnormality in RBC membrane mechanics [Citation15]. Plasma viscosity was measured in a capillary viscometer (Model LBY-N6B, Precil Company, Beijing, China) [Citation16].
Assessment of RBC Deformability
Erythrocyte deformability was quantitated at various shear rates levels using a laser-diffraction ektacytometer system (Model LBY-BX, Precil Company, Beijing, China). This device consists of a laser diode, a concentric cylinder shearing section containing a low-hematocrit suspension of erythrocytes in an isotonic, viscous polymer solution, a stepper motor to rotate the outer cylinder, and a video camera connected to a microcomputer. The microcomputer controls the speed of the motor and analyzes the diffraction resulting when the laser passes through the erythrocyte suspension being sheared. The shape of the laser diffraction pattern is analyzed to obtain an elongation index (EI), determined as: EI = (L 2 W)/(L + W), where L is the length of the image and W is its width [Citation17]. Briefly, 7.5 μL blood was suspended in 1mL 15% polyvinylpyrrolidone (PVP) buffer (Molecular Weight 30 kDa, 61 mM NaCl, 0.8 mM Na2HPO4, 0.2 mM KH2PO4, pH 7.4, 290 mOsm/kg) and was to measure EI at shear rates of 600 s−1, 800 s−1 and 1000 s−1. The EI was determined at 37°C [Citation16].
Determination of RBC Aggregation
RBC aggregation was also determined by the laser-diffraction ektacytometer system (Model LBY-BX, Precil Company, Beijing, China). The measurement is based on the detection of laser back-scattering from the sheared (disaggregated), then unsheared (aggregating) blood, performed in a computer-assisted system at 37°C. Back-scattering data are evaluated by the computer. Aggregation index (AI), amplitude, and top time are calculated on the basis that there is less light back-scattered from aggregating RBCs [Citation17].
Fluid Shear Stress Experiments and Monitoring of RBC Aggregation
Blood samples were drawn from healthy New Zealand White rabbits (weighing 2.5–3.5 kg). Animals were treated according to institutional norms for laboratory animal care. All experiments were approved by the ethics committee of our institute. Blood samples were centrifuged at 1500 × g for 10 min at 4°C. Plasma was withdrawn and RBCs were mixed with plasma and tested solutions to obtain RBC suspensions with a hematocrit of 35% (35% RBC + 35% Plasma + 30% tested solution).
RBC suspensions were exposed to laminar flow for 100s in a parallel plate flow chamber [Citation18] and were driven by a syringe pump (Lange, LEAD-1, Baoding, China). The magnitude of shear rate (γ, in s−1) was calculated according to the formula [Citation19] γ = 6Q/ab2, where Q is volume flow (in mL/sec), a and b are the width and height (in cm) of the flow path, respectively. γ is approximately 1000 s−1 in this study. RBC suspensions were photographed using bright-field light microscopy (OLYPUS IX 71, Japan) before shearing and 2 min after the end of shearing.
Scanning Electron Microscopy
In order to investigate the effect of different tested solutions on RBC aggregation, tested solutions were added to the whole blood obtained from the rabbit (solution: blood in a ratio of 3:7). After 30 min, samples were fixed with 2% glutaraldehyde in a 0.1 M Na-cacodylate buffer with 0.1 M sucrose for 12 h, then treated with filtered 1% tannic acid in a 0.15 M Na-cacodylate buffer for 1 h and post-fixed with 1% osmium tetroxide in 0.1 M Na-cacodylate for 1 h. After this, the fixed cells were dehydrated in graded ethanol solutions, and critical point dried. Imaging was carried out at 0.5 Torr using the low vacuum mode of environment scanning electron microscopy (Quanta 200 FEG, FEI, USA).
Statistical Analysis
Blood samples from five donors were used for each tested solution. Data were demonstrated as mean (SD). Differences between groups were evaluated using ANOVA with DUNNETT test. The statistical differences are considered significant when p < 0.05. The statistical differences are considered strongly significant when p < 0.01. Statistical analysis was performed using the Statistical Analysis System (SAS).
RESULTS
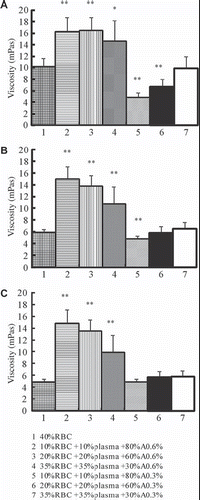
Effects of Hematocrits and the Partial Replacement of Plasma with High-viscosity Solutions on the Viscosity of RBC Suspensions
Viscosities of 40% RBC are 10.17 (SD 1.49), 5.96 (SD 0.50), and 4.96 (SD 0.49) at shear rates of 10 s−1, 60 s−1 and 150 s−1, respectively. As shown in , replacement of plasma with A0.6% at hematocrits of 10% reveals a highly significant increase in viscosity at shear rates of 10 s−1 (P = 0.001), 60 s−1 (P = 1.531 E-5), and 150 s−1 (P = 1.268 E-5) compared to 40% RBC. Replacement of plasma with A0.6% at hematocrits of 20% may contribute to a highly significant increase in viscosity at shear rates of 10 s−1 (P = 0.001), 60 s−1 (P = 1.323 E-5), and 150 s−1 (P = 9.997 E-6) compared to 40% RBC. Replacement of plasma with A0.6% at the hematocrit of 35% may lead to a highly significant increase in viscosity at shear rates of 10 s−1(P = 0.033), 60 s−1 (P = 0.008), and 150 s−1 (P = 0.006) compared to 40% RBC. Replacement of plasma with A0.3% at hematocrit of 10% reveals significant decrease in viscosity at shear rates of 10 s−1 (P = 1.119 E-4) and 60 s−1 (P = 0.010) compared to 40% RBC. Replacement of plasma with A0.3% at hematocrits of 20% reveals a highly significant decrease in viscosity at shear rates of 10 s−1 (P = 0.005) compared to 40% RBC.
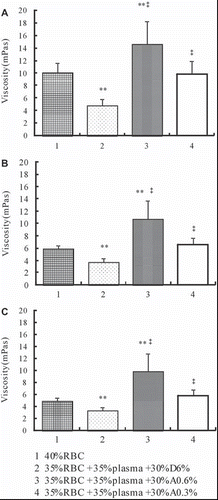
Effects of Different Tested Solutions on Viscosity of RBC Suspensions (Hematocrit of 35%)
As shown in , viscosities of RBC suspensions in which the plasma were replaced with D6% (at the hematocrit of 35%) were 4.86 (SD 0.97), 3.75 (SD 0.70), and 3.34 (SD 0.57) at shear rates of 10 s−1, 60 s−1, and 150 s−1, respectively. Replacement of plasma with D6% reveals a highly significant decrease compared with 40% RBC at shear rates of 10 s−1 (P = 1.560 E-4), 60 s−1 (P = 4.485 E-4), and 150 s−1 (P = 0.001). Replacement of plasma with A0.6% at the hematocrit of 35% reveals a highly significant increase in viscosity compared with D6% at shear rates of 10 s−1 (P = 3.853 E-4), 60 s−1 (P = 0.001), and 150 s−1 (P = 0.001). Replacement of plasma with A0.3% at the hematocrit of 35% reveals a highly significant increase in viscosity compared with D6% at shear rates of 10 s−1 (P = 0.001), 60 s−1 (P = 0.002), and 150 s−1 (P = 0.001).
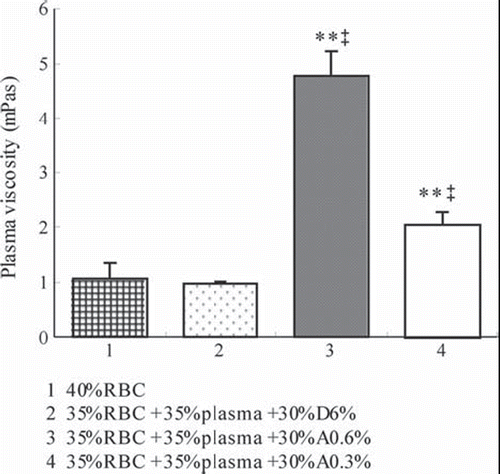
Effects of Different Tested Solutions on Plasma Viscosity
The plasma viscosity of 40% RBC is 1.06 (SD 0.30). As shown in , replacement of plasma with D6% shows no difference, while replacement of plasma with A0.6% (P = 6.49E-14) and A0.3% (P = 1.00E-7) reveals a highly significant increase in plasma viscosity compared to 40% RBC. The plasma viscosity of RBC suspensions whose plasma was replaced with D6% (at the hematocrit of 35%) was 0.97 (SD 0.04). Replacement of plasma with A0.6% (P = 1.54E-14) and A0.3% (P = 1.30E-10) reveals a significant increase in plasma viscosity compared with D6%.
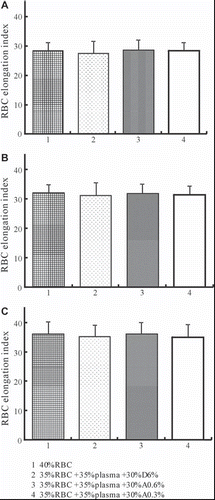
Effects of Different Tested Solutions on RBC Deformability
As shown in , although replacement of plasma with different tested solutions reveals different viscosity, the RBC elongation index that indicates RBC deformability does not cause statistical differences among different tested solutions.
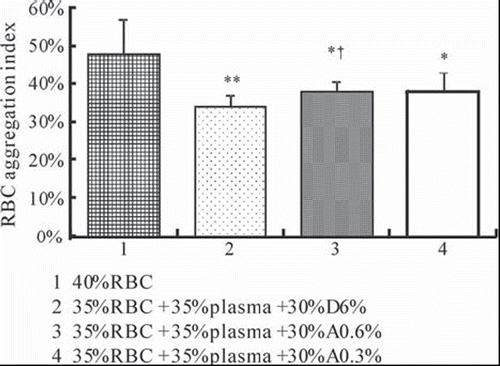
Effects of Different Tested Solutions on RBC Aggregation
The RBC aggregation index of 40% RBC is 48.06% (SD 9.15%). As shown in , replacement of plasma with D6% (P = 0.008), A0.6% (P = 0.025), and A0.3% (P = 0.035) at 35% hematocrit reveals a highly significant decrease in RBC aggregation index compared to 40% RBC. The RBC aggregation index of RBC suspensions whose plasma was replaced with D6% (at the hematocrit of 35%) is 34.06% (SD 2.80%). Replacement of plasma with A0.6% at the hematocrit of 35% reveals a significant increase in RBC aggregation index compared with D6% (P = 0.043). Replacement of plasma with A0.3% at the hematocrit of 35% shows an increase in RBC aggregation index compared with D6%, but the differences are not statistically significant (P = 0.175).
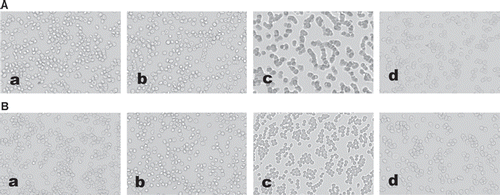
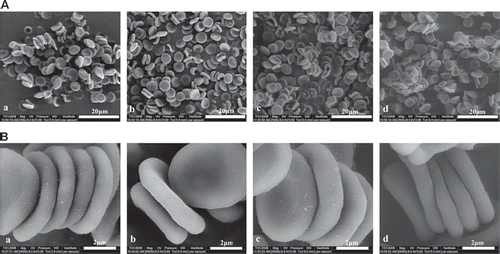
Photographs of RBC aggregation in fluid shear stress experiments are consistent with the determination of RBC aggregation by the laser-diffraction ektacytometer system. 2 min after the end of shearing, 40% RBC and 35% RBC + 35% Plasma + 30% A0.6% show more RBC aggregates than 35% RBC + 35% Plasma + 30% D6% (). Observations by scanning electron microscope show more RBC aggregates after being treated with A0.6% and A0.3% than being treated with D6% ().
DISCUSSION
Alginate is a polysaccharide that has a wide range of applications. It can be used in drug delivery systems and the properties of its solutions and gels suggest biomedical and pharmaceutical uses [Citation20,Citation21]. Alginate can be obtained in an ultrapure form suitable for implants, and transplantation of alginate-based microcapsulated cells is proposed as a therapy for many diseases [Citation22,Citation23]. Although SA of lowered polymerization has been used as a plasma expander in Japan in the 1950s [Citation12,Citation13], alginate material is not presently approved as an injection for human use. SA of a comparatively low concentration can result in high viscosity with a low COP, thus SA of higher polymerization was used as viscogenic material in the study on the effects of HVPE [Citation2,Citation5,Citation6]. Moreover, SA was used as “viscosity modifier” in perfluorocarbon emulsion (A.C. Silva, unpublished observation). We have used SA as “viscosity modifier” in the treatment of experimental hemorrhagic shock [Citation24] and have found that SA of some kinds caused formation of clots in RBC suspensions. Therefore, the effects of SA on the rheology of RBC arouse our interest. To our knowledge, there are few studies about the changes in RBC rheological behaviors caused by SA. In this in vitro study, we used the ultrapure SA as previously reported [Citation2,Citation5,Citation6] to investigate the effect of SA on RBC rheological behaviors.
The rheological behavior of RBCs greatly influences blood circulation both in capillaries and postcapillary venules, as well as transcapillary exchanges [Citation25]. Reduced RBC deformability may adversely affect capillary recruitment and physiological mechanisms that ensure adequate delivery of oxygen to tissue [Citation26]. Induction of RBC aggregation with Dextran 500 at lower arterial pressure can reduce functional capillary density [Citation27]. Besides, enhanced RBC aggregation may relate to decreased wall shear stresses and results in suppressed expression of nitric oxide synthesizing mechanisms, thereby leading to altered vasomotor tonus [Citation28].
The WBV is determined mainly by plasma viscosity, hematocrit and RBC deformability at high shear rates, whereas plasma viscosity mainly depends on the concentration of high-molecular-weight proteins [Citation29]. In the present study, concentrations of SA affected the blood viscosity dramatically. Blood substituted with A0.6% to achieve 10%, 20%, and 35% hematocrits resulted in viscosities significantly higher as compared to the whole blood under 40% hematocrit at all shear rates, while blood substituted with A0.3% to achieve 10% hematocrit resulted in viscosities significantly lower as compared to the blood under 40% hematocrit at shear rates of 10 s−1 and 60 s−1. Blood substituted with A0.3% to achieve 35% hematocrit resulted in viscosities that did not show any differences compared to the blood under 40% hematocrit at all shear rates. At the hematocrit of 35%, A0.3% and A0.6% achieve significantly higher PV compared with D6%.
The WBV, PV, and RBC rheological behaviors of different tested solutions were studied at the hematocrit of 35%, because oxygen transport reaches its maximum value for this hematocrit [Citation30]. Replacement of plasma with A0.6% and A0.3% under a hematocrit of 35% revealed significant increase in WBV and PV compared with D6% at all shear rates; however, RBC deformability did not show any differences among tested solutions. The main parameters that affect RBC deformability are inner viscosity of the cells, surface/volume ratio, and membrane properties related to the lipid bilayer or to the cytoskeleton [Citation31], and the results indicated that neither SA nor D70 affects these properties of RBC dramatically.
RBC aggregation in vivo is mainly determined by plasma protein composition and surface properties of RBC [Citation32]. Dextran 500 can induce RBC aggregation in animal models [Citation27], and dextran was predicted to induce RBC aggregation by two theoretical models: intercellular bridging and depletion layer. In vitro studies have demonstrated that RBC aggregation is caused by the formation of dextran bridges between the cells [Citation33]. It is suggested that the influence of a nonionic polymer or plasma protein on RBC aggregation is simply a consequence of its size in an aqueous environment, and that the specific type of macromolecule is of minor importance [Citation34]. SA is a polyelectrolyte [Citation35] that consists of β (1–4)-linked D-mannuronic acid units and some L-guluronic acid units [Citation36]. SA of different types can be distinguished by their mannuronic acid content and viscosity. In the present study, LVM alginate, whose manuronic acid content was 54%, was used. A0.6% revealed significant increase in RBC aggregation compared with D6%, while A0.3% showed an increase that was not statistically significant. These results indicated that SA may induce RBC aggregation at a much lower concentration compared with D6%, although the relationship between concentrations of SA and formation of RBC aggregates are still to be investigated. Alginate may have mechanisms inducing RBC aggregation different from dextran, because it perfectly performs the function at a much lower concentration, and it may act as an alternative to induce RBC aggregation. It is possible that the mechanisms involve changes of electric properties on the surface of RBC membrane caused by SA, but the detailed mechanisms are still to be investigated.
In addition, RBC morphological imaging is performed to confirm the results obtained by the laser-diffraction method. We have developed a hydrodynamic system that utilizes a parallel-plate flow chamber at a shear rate of 1000 s−1. RBC suspension substituted with A0.6% was found to induce more RBC aggregation. Typical images of RBC aggregation were obtained by scanning electron microscopy.
Plasma expander is known to improve perfusion by increasing blood volume, as well as by improving blood flow, one of whose crucial determinants is the rheological behavior of RBC. There is extensive discussion regarding the effects of RBC aggregation on in vivo blood flow resistance. Some studies have demonstrated increased microcirculatory flow resistance with enhanced RBC aggregation, while whole organ studies revealed that flow resistance may be significantly decreased if RBC aggregation is enhanced [Citation37]. Menu P. et al. found that dextran-benzene-tetra-carboxylate hemoglobin (Dex-BTC-Hb), a kind of plasma expander, had a hyperaggregating effect on RBC, similar to that of some clinically used volume expanders in in vitro study [Citation38,Citation39]. They also found that resuscitation with Dex-BTC-Hb increased WBV at low shear rates as well as RBC aggregation, whereas hydroxyethyl starch 200 decreased WBV but increased RBC aggregation in a rabbit model. These results could contribute to an understanding of the vasoactive effects of Hb-based oxygen carriers [Citation40]. Alginate plasma expander was known to sustain microcirculation during extreme hemodilution and treatment of hemorrhagic shock [Citation2,Citation5,Citation6]. The mechanisms involve elevated plasma viscosity and shear stress that upregulate production of nitric oxide. Due to the results of the present study, the effect of SA on RBC aggregation may be involved in the regulation of microcirculation, and could influence the outcome of extreme hemodilution and hemorrhagic shock. The peripheral resistance of the circulatory system may be altered depending on the rheological properties of the artificial blood substitutes [Citation41]. The effects of plasma expanders or blood substitutes on RBC aggregation, which involve the regulation of microcirculation and influence the clinical treatment, should also be taken into consideration. In vitro study on RBC rheological property is easily carried out and could be a useful method in the development of plasma expanders or blood substitutes. Moreover, in vivo rheological effects may differ significantly from the in vitro effects. The in vitro findings in the present study remain to be determined using animal models in the future.
In summary, this in vitro study compared the effect of high viscosity alginate plasma expander and D70 on RBC rheological behavior. A0.6% elevated WBV at different hematocrits. Replacement of plasma with A0.6% and A0.3% at the hematocrit of 35% revealed significant increase in WBV and PV compared with D6% at all shear rates; however, RBC deformability did not show any differences among tested solutions. A0.6% revealed significant increase in RBC aggregation compared with D6%. SA can be used as an alternative to induce RBC aggregation. The effect of SA on RBC aggregation may be involved in the regulation of microcirculation, and the effects of plasma expanders or blood substitutes on RBC rheological behavior should be taken into consideration.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Tsai, A.G., Intaglietta, M. (2001). High viscosity plasma expanders: Volume restitution fluids for lowering the transfusion trigger. Biorheology 38: 229–237.
- Cabrales, P., Tsai, A.G., Intaglietta, M. (2005). Alginate plasma expander maintains perfusion and plasma viscosity during extreme hemodilution. Am J Physiol Heart Circ Physiol 288: 1708–1716.
- Cabrales, P., Tsai, A.G., Intaglietta, M. (2004). Microvascular pressure and functional capillary density in extreme hemodilution with low- and high-viscosity dextran and a low-viscosity Hb-based O2 carrier. Am J Physiol Heart Circ Physiol 287: 363–373.
- Cabrales, P., Tsai, A.G. (2006). Plasma viscosity regulates systemic and microvascular perfusion during acute extreme anemic conditions. Am J Physiol Heart Circ Physiol 291: 2445–2452.
- Cabrales, P., Intaglietta, M., Tsai, A.G. (2005). Increase plasma viscosity sustains microcirculation after resuscitation from hemorrhagic shock and continuous bleeding. Shock 23: 549–555.
- Cabrales, P., Tsai, A.G., Intaglietta, M. (2004). Hyperosmotic- hyperoncotic versus hyperosmotic-hyperviscous: small volume resuscitation in hemorrhagic shock. Shock 22: 431–437.
- Martini, J., Cabrales, P., Tsai, A.G., Intaglietta, M. (2006). Mechanotransduction and the homeostatic significance of maintaining blood viscosity in hypotension, hypertension and haemorrhage. J Intern Med 259: 364–372.
- Stowell, C.P. (2005). What happened to blood substitutes? Transfus Clin Biol 12: 374–379.
- Tsai, A.G., Acero, C., Nance, P.R., Cabrales, P., Frangos, J.A., Buerk, D.G., Intaglietta, M. (2005). Elevated plasma viscosity in extreme hemodilution increases perivascular nitric oxide concentration and microvascular perfusion. Am J Physiol Heart Circ Physiol 288: 1730–1739.
- Cabrales, P., Tsai, A.G., Intaglietta, M. (2007). Is resuscitation from hemorrhagic shock limited by blood oxygen-carrying capacity or blood viscosity? Shock 27: 380–389.
- Cabrales, P., Intaglietta, M., Tsai, A.G. (2007). Transfusion restores blood viscosity and reinstates microvascular conditions from hemorrhagic shock independent of oxygen carrying capacity. Resuscitation 75:124–134.
- Tomoda, M., Inokuchi, K. (1959). Sodium alginate of lowered polymerization (alginon): A new plasma expander. J Int. Coll. Surg 32: 621–635.
- Murphy, G.P., Mundy, R.L., Ewald, R.A. (1964). The physiologic properties of glyco-alginate, a new Japanese plasma-volume expander. Surgery 56: 1099–1108.
- Ogawa, T. (1973). Post-operative renal insufficiency caused by infusion of alginate plasma expander. Rinsho Byori 21: 395–404.
- Stamos, T.D., Rosenson, R.S. (1999). Low high density lipoprotein levels are associated with an elevated blood viscosity. Atherosclerosis 146:161–165.
- Zhao, L., Wang, B., You, G.X., Wang, Z.L., Zhou, H. (2009). Effects of different resuscitation fluids on the rheologic behavior of red blood cells, blood viscosity and plasma viscosity in experimental hemorrhagic shock. Resuscitation 80: 253–258.
- Erken, G., Bor Kucukatay, M., Erken, H.A., Kursunluoglu, R., Genc, O. (2008). Influence of classical and rock music on red blood cell rheological properties in rats. Med Sci Monit. 14: 28–33.
- Frangos, J.A., Eskin, S.G., McIntire, L.V., Ives, C.L. (1985). Flow effects on prostacyclin production by cultured human endothelial cells. Science 227: 1477–1479.
- Milkiewicz, M., Kelland, C., Colgan, S., Haas, T.L. (2006). Nitric oxide and p38 MAP kinase mediate shear stress-dependent inhibition of MMP-2 production in microvascular endothelial cells. J Cell Physiol 208: 229–237.
- Tønnesen, H.H., Karlsen, J. (2002). Alginate in drug delivery systems. Drug Dev Ind Pharm 28: 621–630. Koppensteiner, R. Blood rheology in emergency medicine. Semin Thromb Hemost 22: 89–91.
- Draget, K.I., Skjåk-Braek, G., Smidsrød, O. (1997). Alginate based new materials. Int J Biol Macromol 21: 47–55.
- de Vos, P., Faas, M.M., Strand, B., Calafiore, R. (2006). Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials 27: 5603–5617.
- Siebers, U., Horcher, A., Bretzel, R.G., Federlin, K., Zekorn, T. (1997). Alginate-based microcapsules for immunoprotected islet transplantation. Ann N Y Acad Sci 831: 304–312.
- Zhao, L., You, G.X., Xu, J., Zhou, H. (2008). Preparation of different viscosities plasma expander and their application in hemorrhagic shock. Chin J Microcirculation 18:10–13.
- Nolte, D., Botzlar, A., Pickelmann, S., Bouskela, E., Messmer, K. (1997). Effects of diaspirin-cross-linked hemoglobin (DCLHb) on the microcirculation of striated skin muscle in the hamster: a study on safety and toxicity. J Lab Clin Med 130: 314–327.
- Parthasarathi, K., Lipowsky, H.H. (1999). Capillary recruitment in response to tissue hypoxia and its dependence on red blood cell deformability. Am J Physiol 277: 2145–2157.
- Kim, S., Popel, A.S., Intaglietta, M., Johnson, P.C. (2006). Effect of erythrocyte aggregation at normal human levels on functional capillary density in rat spinotrapezius muscle. Am J Physiol Heart Circ Physiol 290: 941–947.
- Baskurt, O.K., Yalcin, O., Ozdem, S., Armstrong, J.K., Meiselman, H.J. (2004). Modulation of endothelial nitric oxide synthase expression by red blood cell aggregation. Am J Physiol Heart Circ Physiol 286: 222–229.
- Lowe, G.D. (1987). Blood rheology in vitro and in vivo. Baillieres Clin Haematol 1: 597–636.
- Chien, S. (1982). Rheology in the microcirculation in normal and low flow states. Adv Shock Res 8: 71–80.
- Stoltz, J.F. (1983). Red blood cell microrheology (clinical and pharmacological applications). Ric Clin Lab 13: 53–70.
- Baskurt, O.K., Meiselman, H.J. (2003). Blood rheology and hemodynamics. Semin Thromb Hemost 29: 435–450.
- Pribush, A., Zilberman-Kravits, D., Meverstein, N. (2007). The mechanism of the dextran-induced red blood cell aggregation. Eur Biophys J 36: 85–94.
- Armstrong, J.K., Wenby, R.B., Meiselman, H.J., Fisher, T.C. (2004). The hydrodynamic radii of macromolecules and their effect on red blood cell aggregation. Biophys J 87: 4259–4270.
- Katahaisky, A., Michaell, I. (1955). Polyelectrolyte gels in salt solutions. J Polym Sci 15: 69–86.
- Chanda, S.K., Hirst, E., Percival, E.G.V., Ross, A.G. (1952). Structure of alginic acid. J Chem Soc 1833–1837.
- Baskurt, O.K., Meiselman, H.J. (2007). Hemodynamic effects of red blood cell aggregation. Indian J Exp Bio 45: 25–31.
- Menu, P., Longrois, D., Faivre, B., Donner, M., Labrude, P., Stoltz, J.F., Vigneron, C. (1999). Rheological behaviour of red blood cells suspended in hemoglobin solutions. In vitro study comparing dextran-benzene-tetra-carboxylate hemoglobin, stroma free hemoglobin and plasma expanders. Transfus Sci. 20: 5–16.
- Menu, P., Faivre, B., Labrude, P., Vigneron, C. (1995). In vitro effect of dextran-benzene-tetra-carboxylate hemoglobin on human blood rheological properties. Artif. Cells Blood Substit. Immobil. Biotechnol. 23: 319–330.
- Menu, P., Bleeker, W., Longrois, D., Caron, A., Faivre-Fiorina, B., Muller, S., Labrude, P., Stoltz, J.F. (2000). In vivo effects of Hb solutions on blood viscosity and rheologic behavior of RBCs: comparison with clinically used volume expanders. Transfusion 40(9): 1095–1103.
- Shrinivasan, S., Eggleton, C.D. (2004). Viscosity of animal erythrocyte suspensions mixed with a perflurocarbon emulsion. Artif. Cells Blood Substit. Biotechnol. 32: 387–400.