Abstract
In this study the biosensor was constructed by immobilizing tissue homogenate of banana peel onto a glassy carbon electrode surface. Effects of immobilization materials amounts, effects of pH, buffer concentration and temperature on biosensor response were studied. In addition, the detection ranges of 13 phenolic compounds were obtained with the help of the calibration graphs. Storage stability, repeatability of the biosensor, inhibitory effect and sample applications were also investigated. A typical calibration curve for the sensor revealed a linear range of 10–80 μM catechol. In reproducibility studies, variation coefficient and standard deviation were calculated as 2.69%, 1.44 × 10−3 μM, respectively.
INTRODUCTION
Phenolic compounds (PCs) are produced as secondary metabolites by most plants, in which they probably act as natural antimicrobial agents, as natural deterrents to grazing animals, or as inhibitors of pre-harvested seed germination [Citation1]. Antioxidant compounds such as catechin, rutin, and quercetin, which exist in fruits, have been claimed to protect low density lipoproteins and have anti-cancer effects [Citation2]. Polyphenols are also important for their organoleptic properties, odors, astringency, and savor for wine, tea and other fruits [Citation3]. Furthermore, PCs are also formed during the natural decomposition of humic substances, tannins, and lignins, and photolytic or metabolic degradation of herbicides and insecticides [Citation4, Citation5]. More recently, the significance of PCs as antioxidants, anticarcinogens, antimutagens and agents in the treatment of Rh-factor-threatened pregnancies, problems encountered during parturition, and AIDS has received a lot of research attention [Citation6,Citation7,Citation8]. Therefore, sensitive, rapid and precise determination of PCs has created growing interest in the environmental control, protection, and health benefits [Citation9,Citation10]. Colorimetric and ultraviolet spectrophotometric analyses [Citation11,Citation12], high performance liquid chromatography [Citation13], gas chromatography [Citation14], and capillary electrophoresis [Citation15] are now commonly used for the determination of PCs as standard methods. However, these schemes do not easily allow continuous on-site monitoring, they have high costs, are time-consuming, they need skilled operators, and also sometimes require preconcentration and extraction steps. To solve this problem, a simple, effective and fast alternative method for the determination of phenolic compounds is desirable.
Amperometric biosensors for PCs based on polyphenol oxidase are proving to be promising for this purpose [Citation11, Citation16]. Biosensors based on pure enzymes may provide a promising method for a simple, fast and sensitive detection of phenolic compounds. However, many of them have a limited lifetime due to enzyme inactivation by the biocatalytically generated quinine products. Thus, development of good immobilization methods and materials to improve the biosensor stability is very significant [Citation17, Citation18]. For determination of phenolic compounds, biosensors modified with tyrosinase [Citation19, Citation20, Citation21], peroxidase [Citation22, Citation23, Citation24], and laccase [Citation25, Citation26, Citation27] have been reported. Plant and animal tissues have been successfully employed as biocatalytic components in the construction of biosensors for about two decades. When biosensors are compared with immobilized isolated and pure enzymes, tissue-based biosensors show potential advantages of low cost, high stability, longer lifetime, and high level activity. Since 1981, when the first plant tissue-based electrode was reported, immobilization of polyphenol oxidase (PPO) with some plant tissue homogenate for use in determination of phenolic compounds was performed by dissolved oxygen probe [Citation28, Citation29]. However, in this article, PPO within banana peel tissue homogenate was immobilized on a glassy carbon electrode (GCE).
In this study, we describe a new amperometric biosensor for the determination of PCs based on banana peel (Musa cavendish) plant tissue. The enzyme PPO in the peeled banana (Musa cavendish) has been characterized in a previous study but the enzyme PPO in the banana peel (Musa cavendish) has not been characterized in the literature yet [Citation30]. Optimization and characterization studies of the new biosensor based on banana peel were carried out. For each phenolic substrate, the calibration graphs were drawn and detection limits were determined using the biosensor. Inhibitor studies and application for determination of the phenolic compounds in some drinks were also performed.
MATERIALS AND METHODS
Reagents
All reagents used were of analytical grade. Buffer solutions were prepared using distilled water. The banana peel used was purchased from Anamur-Mersin grocery. KH2PO4, K2HPO4, phenol, Na2HPO4, NaH2PO4, hydroquinone, pyrogallol, catechol, ascorbic acid, thiourea, benzoic acid, CuSO4, ZnSO4, and HgCl2 were purchased from E. Merck (Germany). Glutaraldehyde (25 %), sodium sulfite, sodium bisulfide, cysteine, gelatin (225 Bloom), glycine, L-Dopa, p-cresol, gallic acid, caffeic acid, rutin hydrate, 4 hydroxy cinnamic acid, resorcin, and quercetin were purchased from Sigma (St. Louis, MO, USA).
Apparatus
In this study, a potentiostat (Palmsense, Netherlands), glassy carbon working electrode, Pt counter electrode, and Ag/AgCl reference electrodes (Basi, W. Lafayette, USA) were used for determination of the flowing current level. All the measurements were carried out at a constant temperature using a thermostat (Nüve BM 302, UK). To prepare the buffer solution a magnetic stirrer (RCA, UK) and pH meter with an electrode (Schott Handlylab, Spain) were used. The temperature in the reaction cell was maintained unchanged by circulating water at an appropriate temperature around the cell compartment during the experiment.
Procedure
Preparation of the bioactive layer material. 165 mg of banana peels were weighed and homogenized with 550 μL working buffer (66 mM, pH 7, phosphate buffer) by a manual glass homogenizer. Freshly prepared tissue samples were used for each day.
10 mg gelatin was weighed and added to a test tube. Banana peel tissue homogenate (300 μL) was added into the test tube. The mixture of banana peel homogenate and gelatin was incubated at 38°C for 15 min to dissolve gelatin.
Preparation of the biosensor. Gelatin-banana peel tissue (30 μL) homogenate was dispersed over the cleaned GCE surface and allowed to dry at 4°C for 30 min. For cross linking with glutaraldehyde, the probe-carrying bioactive layer was immersed into 5% (v/v) glutaraldehyde solution (in phosphate buffer, 20 mL, 66 mM, pH 7) and was allowed to stay for 5 min. Then the biosensor was washed with distilled water to remove excess glutaraldehyde and it was ready to use.
After it was used, the biosensor was stored at 4°C in a flask that contained distilled water. The biosensor did not have contact with the water. This condition provided a moisture medium; therefore the dryness of the bioactive layer was prevented.
Measurement procedure. The GCE based on banana peel tissue homogenate, Pt counter electrode, and Ag/AgCl reference electrode were put into the thermostatic reaction cell containing the working buffer (pH 7.0, 66 mM sodium phosphate buffer) and the magnetic stirrer was fixed at a constant speed. Then −0.700 mV potential was applied for the reduction of the oxygen on the tissue-modified GCE surface. A few minutes later, the current of the system was equilibrated because of the diffusion of dissolved oxygen between the working buffer and tissue-modified GCE. At this time, the phenolic compound was injected into the thermostatic reaction cell. The flowing current started to decrease and, a few minutes later, it reached the constant value due to the enzymatic reaction equilibration below.
At this moment, constant current was recorded. Electrical response, which constituted the output signal from the biosensor, was acquired using the PalmsensePC software package, purchased from the Palm Instrument PV (Ivium Technologies, Netherlands). Measurements were carried out by noting the decrease of current in relation to the phenolic substrate concentration added into the reaction cell.
RESULTS AND DISCUSSION
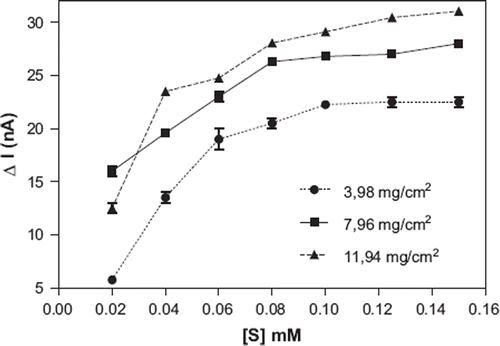
Effect of the Banana Peel Tissue Amount
The amount of tissue on GCE surface changed as shown in for the determination of the effect of tissue amount on the biosensor response. As is obvious from the figure, the optimum amount of the tissue was 7.96 mg/cm2 because of better linear range and higher response than 3.98 mg/cm2 and 11.94 mg/cm2 tissue. Tissue amounts of tissue-based biosensors have a wide range. Optimum amount of tissue on the electrode surface depends on amounts and types of enzymes in different tissues. For example, optimum tissue amount of mushroom and Jerusalem artichoke-based biosensors were given as 44.25 mg/cm2 and 98 mg/cm2, respectively [Citation28, Citation31].
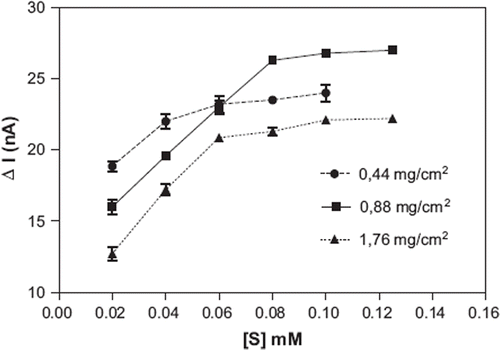
Effect of the Gelatin Amount
Experiments were carried out by keeping the amount of banana peel tissue and percentage of glutaraldehyde constant, in order to investigate the effect of differing gelatin amounts on the GCE surface. Results obtained are given in .
As can be seen in , the highest biosensor response was observed when 0.88 mg/cm2 gelatin was used. Same results were reported for gelatin-based biosensors in the literature. For example, optimum gelatin amounts of spinach- and cucumber-based biosensors were given as 0.89 mg/cm2 and 0.70 mg/cm2, respectively [Citation32,Citation33].
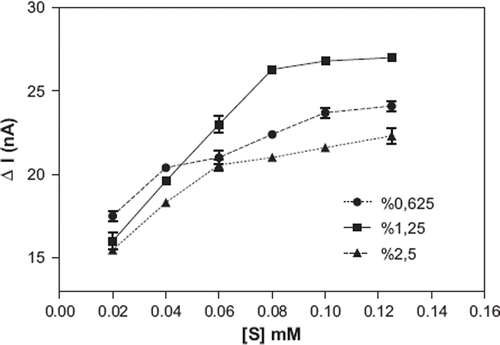
Effect of the Glutaraldehyde Percent
For the determination of the effect of glutaraldehyde percent on the biosensor response, banana peel tissue amount and gelatin amount were kept constant and we changed the glutaraldehyde percent in the experiments. As can be seen in , the optimum glutaraldehyde percent was 1.25 %. The used percentage of the glutaraldehyde for construction of a tissue-based biosensor may change according to the type of tissue and immobilization material and also the surface area of the electrodes. A similar result was reported for Malva vulgaris based biosensor [Citation34].
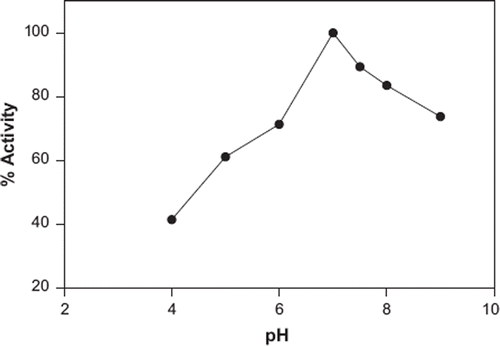
pH Effect on the Biosensor Response
The activity of the biosensor as a function of pH when catechol is used as a substrate is shown in . The citric acid buffer used was pH 4, phosphate buffer used was in the pH range from 5–8, and glycine buffer used was pH 9. It is seen that there are decreases in the activity of the biosensor in the pH range from 4–6 and 7.5–9. The highest activity was obtained at pH 7. PPO enzymes where found in various plants have optimum pH range of 5–8. This result is compatible with the literature [Citation35].
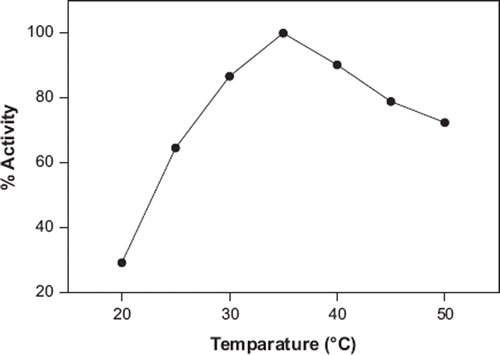
Effect of the Temperature
The effect of assay temperature (20–50°C) was examined by using catechol. As is shown in , the maximum sensor response was found at 35°C. Therefore, this temperature was accepted as optimum temperature for all following experiments. PPO enzymes in various plants show maximum activity at a range of 25–40°C. The same result (35°C) was given for mushroom-based biosensors for determination of phenolic compounds [Citation35, Citation36].
The Effect of the Buffer Concentration on the Biosensor Response
The increase of buffer concentration contributes to the reproducibility of biosensor response together with the decay of the response value and the lifetime of the immobilized enzyme or tissue. Because of this, we also investigated optimum concentration of the working buffer. According to the results obtained by testing three different buffer concentrations, 33, 66, and 132 mM, the highest biosensor response was obtained in 66 mM sodium phosphate buffer.
Linear Range
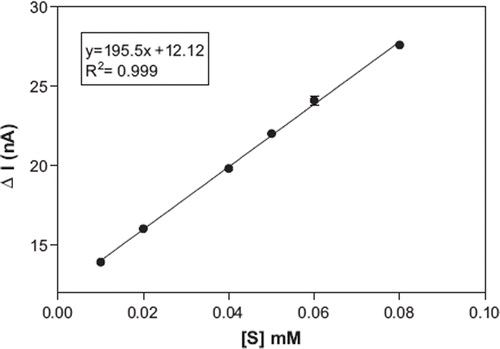
shows the calibration curve of the biosensor based on banana peel tissue for catechol. A linear graph, defined by the equation y = 195.5 x + 12.12, (R2 = 0.999), was obtained. Linearity was found in the range of 10–80 μM. At higher concentrations, the standard curve showed a deviation from linearity. The deviation was due to the limited amount of the polyphenol oxidase in the bioactive material or insufficient dissolved oxygen, which is a co-substrate of the enzyme. The minimum detectable concentration of the catechol was estimated to be 10 μM.
Reproducibility
The reproducibility of the biosensor was tested by 6 average standard solutions containing equal amounts of catechol (50 μM). The standard deviation (SD), variation coefficient (cv), and average value (X) were calculated as 1.44 × 10−3μM, 2.69 % (n=6) and 49.75 μM, respectively.
Storage Stability
For this purpose, the long-term performance of the biosensor was evaluated intermittently over a period of 24 days by monitoring its response to standard catechol solution. The biosensor was stored as described in the biosensor preparation section above. Although this condition provides a moisture medium for the biosensor, it is also possible to prevent drying bioactive layer of the biosensor. In this condition, after a five-day storage period, the biosensor didn't lose any activity at the end of the 6th, 10th, 17th, 20th, and 24th days; the remaining activities of the biosensor were 95.7, 83.2, 78.3, 76.4, and 74.6 %, respectively. If the biosensor lifetime was compared with the other biosensor constructed using glutaraldehyde, gelatin and tissue homogenate by Sezginturk et al. [Citation34] (10 days), it was found that the storage stability of the biosensor is good. Also, Portaccio et al. [Citation26] and Eggins et al. [Citation37] have reported 10 days and 21 days lifetime for phenolic compounds detection by biosensors, respectively.
Inhibitory Effect
In order to show the inhibitor effect on the biosensor-based banana peel, some metal ions and certain substances were tested. Catechol is used as a substrate and inhibitors tested were of 20 μM and 10 μM, respectively. After electrodes were placed in the thermostatic reaction cell and the inhibitor solution was added, −0.700 mV potential was applied. When the flowing current reached a constant value, catechol was injected into the reaction cell. The flowing current started to decrease and a few minutes later it reached the constant value, but not as much when the measurement had been done without the inhibitor. Inhibition studies were carried out by the change of flowing current. The results of the inhibition studies are shown in . As is seen in , Hg+2 have the highest inhibitory effect and Zn+2 have the lowest inhibitory effect on the biosensor. Benzoic acid and thiourea have showed lower inhibitory effect than the results reported for tissue-based biosensor for determination of phenolic compounds by Sezginturk et al. [Citation31]. The results presented show that the banana-peel-based biosensors were not significantly affected by any inhibitor for PPO enzymes.
Table 1. Inhibition studies
Determination of Some Phenolic Compounds
The biosensor was applied to twelve phenolic compounds and ascorbic acid. Analytical results for each compound, obtained by the experiments, are summarized in .
Table 2. Results obtained from the measurements of the some phenolic compounds
As seen in , caffeic acid showed the highest activity. The activity of pyrogallol was 15.9 % lower than the activity of catechol. Pyrogallol activity was followed by cinnamic acid, hydroquinone, catechol, p-cresol, ascorbic acid, gallic acid, resorcin, phenol, rutin, quercetin, and L-dopa. Although ascorbic acid was not a phenolic compound, the response obtained by the biosensor for ascorbic acid was 4.9%, which is less than the response of catechol. This probably resulted from the universal interference effect of ascorbic acid. Moreover, it may result from the cyclic form of ascorbic acid in an aqueous solution.
Sample Applications
The tissue biosensor based on banana peel was applied using a standard addition method in some drink samples. The samples, which included a known amount of catechol (100 μM), were used as stock substrate solutions with different dilution ratios derived by working buffer and were added into the reaction cell, equilibrated, and then the change in current was measured. The signals derived from the drink samples were found to be very similar with the standard catechol solutions having the same concentration. Results are expressed as mean ± S.D, (n=5). The results of the measurements of drink samples are shown in . As is seen in , the biosensor response is not influenced by alcohol and sugar during the measurements of PCs in beer, wine, and fruit juice.
Table 3. Phenolic compound measurements in some drink samples
CONCLUSION
Our observations showed that the biosensor based on banana peel tissue developed in this study could be a good alternative detection method without requiring sample pretreatment. Moreover, sugar content or the alcoholic nature of the samples did not interfere with our measurements. The biosensor is able to measure phenolic compounds, such as caffeic acid, pyrogallol, cinnamic acid, hydroquinone, catechol, p-cresol, ascorbic acid, gallic acid, resorcin, phenol, rutin, quercetin and L-dopa, and the biosensor will be useful for studies seeking to understand the total level of these compounds in some products. All the measurements showed that the biosensor obtained could be used simply and rapidly, and it has the advantage of being inexpensive. The total analysis time of a measurement takes 10-12 min. The proposed biosensor can analyze thirteen kinds of PCs by using their standard curves. However, in a real drink sample such as wine and fruit juice, it is more difficult to detect phenolic compounds separately. In these samples it is possible to analyze total phenolic compounds by the banana peel tissue-based biosensor. Moreover, we anticipate that this biosensor can be used not only for the analysis of phenolic compounds but also for monitoring certain substances which inhibit the PPO in the bioactive material. This study also demonstrates the successful application of a plant tissue on GCE for construction of a biosensor by using uncharacterized tissue (banana peel) in the literature.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Bravo, L. (1998). Polyphenols: chemistry, dietary sources, metabolism and nutritional significance. Nut. Rev. 1 56: 317–333.
- Goldberg, D.M., Tsang, E., Karumanchiri, A., Diamandis, E.P., Soleas, G., Ng, E. (1996). Method to assay the concentration of phenolic constituents of biological interest in wine. Anal. Chem. 68: 1688–1694.
- Romani, A., Minunni, M., Mulinacci, N., Pinelli, P., Vincieri, F.F., Del Carlo, M., Mascini, M. (2000). Comparison among differential pulse voltammetry, amperometric biosensor, and HPLC/DAD analysis for polyphenol determination. J. Agric. Food Chem. 48 (4): 1197–1203.
- Masque, N., Pocurull, E., Marce, R.M., Borrull, F. (1998). Determination of eleven priority EPA phenolics at ng L–1 levels by on-line solid-phase extraction and liquid chromatography with UV and electrochemical detection. Chromatographia. 47: 176–182.
- Narang, A.S., Vernoy, Ch.A., Eadon, G.A. (1983). Evaluation of Nellson–Kryger steam distillation technique for recovery of phenols from soil. J. Assoc. Off. Anal. Chem. 66: 1330.
- Singleton, V.L. (1981). Naturally occuring food toxicants: phenolic substances of plant origin common in foods. Adv. Food Res. 27: 149–242.
- Mukhtar, H., Wang, Z.Y., Katiyar, S.K., Agarwal, R. (1992). Tea components: Antimutagenic and anticarcinogenic effect. Preventative Med. 21: 351–360.
- Chung, K.T., Wei, C.I., Johnson, M.G. (1998). Are tannins a double-edged sword in biology and health. Trends Food Sci. Technol. 9: 168–175.
- Rajesh, Takashima W., Kaneto, K. (2004). Amperometric phenol biosensor based on covalent immobilization of tyrosinase onto an electrochemically prepared novel copolymer poly (N-3-aminopropyl pyrroleco-pyrrole) film. Sens. Actuators B: Chem. 102: 271–277.
- Yu, J., Liu, S., Ju, H. (2003). Mediator-free phenol sensor based on titania sol–gel encapsulation matrix for immobilization of tyrosinase by a vapor deposition method. Biosens. Bioelectron. 19: 509–514.
- Townshend, A. (1995). Encyclopedia of Analytical Science, vol. 8, Academic Press, London, 3928.
- Vogel, V. (1989). Textbook of Quantitative Chemical Analysis, Longman, UK, 707.
- Fiehn, O., Jekel, M. (1997). Analysis of phenolic compounds in industrial wastewater with high-performance liquid chromatography and post-column reaction detection. J. Chromatogr. A 769 (2): 189–200.
- Kishi, H., Arimoto, H., Fujii, T. (1998). Analysis of alcohols and phenols with a newly designed gas chromatographic detector. Anal. Chem. 70: 3488–3492.
- Morales, S., Cela, R. (2000). Highly selective and efficient determination of US Environmental Protection Agency priority phenols employing solid-phase extraction and non-aqueous capillary electrophoresis. J. Chromatogr. A 896: 95–104.
- Ozsoz, M., Erdem, A., Kilinc, E., Gokgunnec, L. (1996). Mushroom based cobalt phthalocyanine dispersed amperometric biosensor for the determination of phenolic compounds. Electroanal. 8: 147–152.
- Mokower, A., Eremenko, A.V., Streffer, K., Wollenberger, U., Scheller, F. W. (1996). Tyrosinase-glucose dehydroenase substrate-recycling biosensor: a highly sensitive measurement of phenolic compounds. J. Chem. Tech. Biotechnol. 65: 39–44.
- Rogers, K.R. (1995). Biosensors for environmental applications. Biosens. Bioelectron 10: 533–541.
- V′edrine, C., Fabiano, S., Tran-Minh, C. (2003). Amperometric tyrosinase based biosensor using an electrogenerated polythiophene film as an entrapment support. Talanta 59: 535–544.
- Campuzano, S., Serra, B., Pedrero, M., Villena, F.J.M, Pingarron, J.M. (2003). Amperometric flow-injection determination of phenolic compounds at self-assembled monolayer-based tyrosinase biosensors. Anal. Chim. Acta 494: 187–197.
- Yu, J., Ju, H. (2004). Pure organic phase phenol biosensor based on tyrosinase entrapped in a vapor deposited titania sol–gel membrane. Electroanalysis 16 (16): 1305–1310.
- Imabayashi, S., Kong, Y.T., Watanabe, M. (2001). Amperometric biosensor for polyphenol based on horseradish peroxidase immobilized on gold electrodes. Electroanalysis 13 (5): 408–412.
- Rosatto, S.S., Kubota, L.T., Oliveira Neto, G. (1999). Biosensor for phenol based on the direct electron transfer blocking of peroxidase immobilising on silica–titanium. Anal. Chim. Acta 390: 65–72.
- Yang, S., Li, Y., Jiang, X., Chen, Z., Lin, X. (2006). Horseradish peroxidase biosensor based on layer-by-layer technique for the determination of phenolic compounds. Sens. Act. B. 114: 774–780.
- Timur, S., Pazarlioglu, N., Pilloton, R., Telefoncu, A. (2004). Thick film sensors based on laccases from different sources immobilized in polyaniline matrix. Sens. Actuators B: Chem. 97: 132–136.
- Portaccio, M., Martino, S.D., Maiuri, P., Durante, D., Luca, P.D., Lepore, M., Bencivenga, U., Rossi, S., Maio, A.D., Mita, D.G. (2006). Biosensor for phenolic compouns: The catechol as a substrate model. J. of Mol. Cataly. B: Enz. 41: 97–102.
- Gomes, S.A.S.S., Nogueira, J.M.F., Rebelo, M.J.F. (2004). An amperometric biosensor for polyphenolic compounds in red vine. Biosens. Bioelect. 20: 1211–1216.
- Odaci, D., Timur, S., Telefoncu, A. (2004). Immobilized Jerusalem artichoke (Helianthus tuberosus) tissue electrode for phenol detection. Art. C. Blo. Subs. Biotec. 32 (2): 315–323.
- Topcu, S., Sezgintürk, M.K., Dinçkaya, E. (2004). Evaluation of a new biosensor-based mushroom (Agaricus bisporus) tissue homogenate: investigation of certain phenolic compounds and some inhibitor effects. Biosens. Bioelect. 20: 592–597.
- Unal, M.U. (2007). Properties of polyphenol oxidase from Anamur banana (Musa cavendish). Food. Chem. 100 (3): 909–913.
- Sezgintürk, M.K., Dinçkaya, E. (2004). Evaluation of a new biosensor-based mushroom (Agaricus bisporus) tissue homogenate: investigation of certain phenoliccompounds and some inhibitor effects. Biosensors & Bioelectronics. 20: 592–597.
- Sezgintürk, M.K., Dinçkaya, E. (2004). An amperometric inhibitor biosensor for the determination of reduced glutatione (GSH) without any derivatization some plants. Biosensors – Bioelectronics. 19: 835–841.
- Sezgintürk, M.K., Dinçkaya, E. (2003). A novel amperometric biosensor based on spinach (Spinacia oleracea) tissue homogenate for urinary oxalate determination. Talanta. 59: 545–551.
- Sezgintürk, M.K., Dinçkaya, E. (2004). Direct determination of sulfite in food sampels by a biosensor based on plant tissue homogenate. Talanta. 65: 998–1002.
- Vamos-Vigyazo, L. (1981). Polyphenol oxidase and preoxidase in fruits and vegetables. CRC Critical Reviews in Food Science and Nutrition. 1: 49–127.
- Teke, M., Sezgintürk, M.K., Dinçkaya, E., Telefoncu, A. (2004). Two biosensors for phenolic compounds based on mushroom (agaricus bisporus) homogenate: comparison in terms of some important parameters of the biosensors, Preparative Biochemistry & Biotechnology. 1: 51–60.
- Eggins, B.R., Hickey, C., Toft, S.A., Zhou, D.M. (1997). Determination of flavanols in beers with tissue biosensors, Anal. Chim. Acta 347: 281–288.