Abstract
Amperometric biosensors using laccase from Trametes versicolor as a bioelement were developed for 2,4-dichloro phenoxy acetic acid (2,4-D). Laccase enzyme was immobilized by gelatin and glutaraldehyde onto a Clark oxygen probe and screen printed electrodes (SPEs). Amperometric and chronoamperometric measurements were carried out with the biosensors. First, the effect of laccase activity on the biosensor performances was investigated for both biosensors, and then optimum pH and temperature and also thermal stability of the biosensors were tested. In addition, the detection ranges of some phenolic compounds were obtained by the help of calibration graphs of them. In repeatability studies, variation coefficients and standard deviations for both biosensors were also calculated by the studies done for this purposes. Finally, the biosensors were applied to the determination of 2,4-D in a real herbicide sample.
INTRODUCTION
Laccases (benzenediol:oxygen oxidoreductases, E.C 1.10.3.2) are a diverse group of multi-copper proteins that catalyze the oxidation of a variety of aromatic compounds. Because of their broad substrate specificity, laccases harbor great biotechnological potential [Citation1–4]. The catalytic properties of laccases have had a great impact on the development of biosensors for both environmentally important pollutants and clinically relevant metabolites [Citation5]. Laccases have been found exclusively in plants and fungi; however, only the fungal laccases are subjects of current biotechnological applications. Fungal laccases are extracellular soluble proteins [Citation6,Citation7]. Non-phenolic substrates can be oxidized by laccase in the presence of mediators [Citation8–12]. The basis of the laccase–mediator concept is the use of low molecular mass compounds that are converted into stable radicals by means of enzymatic oxidation. They act as redox mediators and oxidize other compounds that, in principle, are not substrates of laccase [Citation13]. A number of synthetic organic and inorganic mediators have been found, described, and patented, and naturally occurring “native” mediators for laccases have been discovered and identified [Citation14]. The range of substrates oxidized by laccases can be increased through a mediator-involved reaction mechanism. Mediators are low molecular weight compounds (as syringaldazine) that are easily oxidized by laccases producing, in some cases, very unstable and reactive cationic radicals, which can oxidize more complex substrates before returning to their original state [Citation15].
Chlorinated phenoxyacids are a kind of compound widely used in agriculture. Monitoring of these herbicides is very important in surface water because of their potential toxicity towards animals and humans. 2,4-dichloro phenoxy acetic acid (2,4-D) used to control broad-leafed weed is a member of the chlorophenoxyacetic acid herbicides. Because these compounds contain chlorine, they pose a risk for dioxin formation [Citation16, Citation17]. Exposure of humans and animals occurs through contaminated air, drinking water, soil, and foodstuff during production of the herbicide. 2,4-D may cause a health risk; definitive data are not available concerning its carcinogenity, mutagenity, and genotoxicity [Citation18–22]. In Japan, the tolerance levels of 2.0 μg/g for 2,4-D (0.2 μg/g for DDVP, 4.0 μg/g for malathion, 1.0 μg/g for carbaryl) have been established in citrus fruit [Citation23]. Therefore, sensitive analytical methods for the routine determination of 2,4-D in water and soil samples are highly desired. However, this compound is analyzed separately by liquid chromatography (LC) or gas chromatography (GC) because of its physicochemical properties. On the other hand, 2,4-D and carbaryl are analyzed by GC/MS [Citation24–27] or an LC-fluorometric detector [Citation28] after derivatization. Thus, it is difficult to analyze simultaneously this herbicide in samples by conventional analytical methods such as chromatography. Furthermore, those methods require labor-intensive sample cleanup, strictly controlled chromatographic conditions, and a long separation time. Apart from these classical analytical methods for 2,4-D determination, ELISAs [Citation29–31], disposable amperometric immunosensors [Citation32, Citation33], and a FIA system with optical (fluorometric) detection have been described [Citation34]. Consequently, there is an urgent need for a simple analytical method for the rapid and simultaneous determination of 2,4-D at concentrations near its tolerance levels in samples.
In this study, Trametes versicolor laccase was used to develop biosensors for the determination of exogen xenobiotic 2,4-D as well as some phenolic compounds. For these purposes, two biosensors were constructed using two different kinds of transducer systems. Also, the performances of the biosensors were compared with each other.
EXPERIMENTAL
Chemicals
Chemicals were obtained from either E. Merck (Germany) or Sigma-Aldrich Chemical Co. (USA) as the analytical grades.
Apparatus
WTW Inolab Oxi Level 2 model oxygenmeter based on amperometric mode was used for the experiments. All signals were recorded as dissolved oxygen level (mg/L). Chronoamperometric measurements were performed using a PALM SENS electrochemical measurement system from PALM Inst. B.V. (Netherlands). A water bath was used for preparation of bioactive material (Stuart Sci. Linear Shaker bath SBS 35) (UK). All the measurements were carried out of constant temperature using a thermostat. Magnetic stirrer (IKA-Combimag, RCO) and pH-meter with electrode (WTW pH 538, Germany) for preparing buffer solutions were used. The temperature was maintained constant in the reaction cell by circulating water around the cell during the experiment.
Inks for printing working electrodes were prepared by using a commercially available carbon ink (Du Pont 7101). Printed electrodes were fabricated by depositing several layers of inks on a PVC substrate. The conducting paths and pads were deposited directly on the PVC sheets using Ag/Pd ink (Du Pont 5025). Ag/AgCl ink was deposited to obtain the reference electrode. Carbon ink was printed to obtain the working electrodes. Finally, an insulator layer was placed over the conducting paths.
Procedure
Biological material. TvL was isolated from the culture filtrates of the white rot fungus T. versicolor (ATCC 11 235). T. versicolor was maintained at 4°C on 2% malt agar and grown in 100 ml malt extract broth (2%) for 3 days. The laccase-production medium was a nitrogen-limited medium consisting of 10 g glucose, 1 g NH4H2PO4, 0.05 g MgSO4·7H2O, 0.01 g CaCl2 and 0.025 g yeast extract, per liter. The cultures of T. versicolor were incubated at 26°C on a rotary shaker at 175 rpm. After 72 h cultivation, growing medium was used as a source of enzyme. Laccase production was assessed by measurement of enzyme oxidation of 2,2-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid) at 427 nm (ε = 3.6 × 104 cm−1 M−1) [Citation13]. The reaction mixture contained 300 μL of extracellular fluid, 300 μL of 1 mM ABTS and 0.1 M Na-acetate buffer (pH 4.5). 1.0 Unit of enzyme activity is defined as the amount of enzyme that oxidises 1 μmol ABTS in 1 min. Final activity for TvL was 350 U ml−1.
Preparation of the biosensors. Both dissolved oxygen probe (Type I) and SPE (Type II) surfaces were used for immobilization of laccase. In the step of preparation of the bioactive layer material, laccase (4 U) and 10 mg gelatin were weighed and added to a test tube contained 250 μL of phosphate buffer (pH 7.5, 50 mM). This mixture was incubated at 38°C for 5–10 minutes to dissolve the gelatin completely. For construction of the biosensor based on dissolved oxygen probe, a 250 μL of the laccase-gelatin mixture was dispersed over the dissolved oxygen probe membrane surface and allowed to dry at 4°C for an hour. Then for crosslinking with glutaraldehyde, the probe carrying bioactive layer was immersed into 2.5% (v/v) glutaraldehyde solution (in phosphate buffer, pH 7.5, 50 mM) for 5 minutes. At the end of this period, the electrode was washed with distilled water. For construction of the biosensor based on SPE was the same as for the dissolved oxygen probe, except 5 μL of laccase-gelatin mixture was placed on graphite working electrode and 0.25% (v/v) glutaraldehyde solution (in phosphate buffer, pH 7.5, 50 mM) was used for 150 seconds. In order to prevent drying out of the bioactive layer of the biosensor, it was stored in a flask that contained some distilled water at 4°C. The biosensor was not in contact with distilled water. This condition provided a moisture medium for the biosensor.
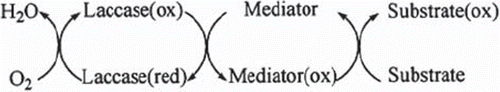
Measurement procedure. For a type I biosensor, the working buffer (10 μM hydroxy benzotriazole (HBT) + pH 4.5, 50 mM acetate buffer) was put into the thermostatic reaction cell. Then the biosensor was put into the reaction cell and the magnetic stirrer was fixed at a constant speed. A few minutes later, dissolved oxygen concentration was equilibrated because of the diffusion of dissolved oxygen between the working buffer and dissolved oxygen probe. At this time, standard or sample was injected into the thermostatic reaction cell. The dissolved oxygen concentration started to decrease and a few minutes later it reached the constant dissolved oxygen concentration. At this moment, dissolved oxygen concentration was recorded. Measurements were carried out by the change of dissolved oxygen concentration related to standard or sample added to the reaction cell (ΔDO, the difference in dissolved oxygen concentrations, mg/L). For a type II biosensor, in order to determine the concentration of substrates oxygen consumption that occurred in the catalytic cycle of a laccase-mediator oxidation system was followed ().
All the measurements were done in the presence of 10 μM of hydroxy benzotriazole as a mediator and at 35°C. The working buffer (10 μM HBT + pH 4.5, 50 mM acetate buffer) was put into the thermostatic reaction cell. Then the biosensor was put into the reaction cell and magnetic stirrer was fixed at a constant speed. A few minutes later, current density was equilibrated, at this time standard or sample was injected into the reaction cell. After substrate addition, its oxidation took place in the bioactive layer and was sensed as a change in the current intensity with a potentiostat at −0.7 V versus Ag/AgCl reference electrode, chronoamperometrically.
RESULTS AND DISCUSSION
Optimization Studies of the Biosensors
Effect of the laccase activity. In these studies, the effect of laccase activity on the biosensors (type I and type II) was investigated. Measurements were accomplished by using each of the standard curves obtained under the conditions below; for type I, the activities of laccases were altered as following 2 U, 4 U, and 8 U while gelatin amount and glutaraldehyde percentage were kept constant as 10 mg and 2.5%, respectively. The results showed that enzyme activity affected slightly the biosensor response. When we used 4 U laccase, we obtained the highest signals from the biosensor. The sensitivity of the biosensor was related to amplitude of the signals. That is to say that if the signals are how much great the detection limit of the biosensor is so much low. As can be seen from , more increase in laccase activity caused a decrease in biosensor signals. The decrease was probably caused from the negative diffusion effect of more protein. Last, the biosensor constructed using 2 U laccase didn't show linear range like that of 4 U laccase. As a result, the best signals were obtained with the biosensor containing 4 U of laccase activity (). Moreover, for type II, the same experiments were done. In these studies, gelatin amount and glutaraldehyde percentage were also kept constant as 0.2 mg and 2.5%, respectively. The best results were obtained by using 4 U activity of laccase as the same with the type I biosensor.
Figure 2. Laccase activity optimization [Laccase activities: –•–•–: 4 U, –▪–▪–: 8 U, –▴–▴–: 2 U. Working conditions: Amount of gelatin and percentage of glutaraldehyde were kept constant as 10 mg and 2.5%, respectively. Working buffer: pH 4.5, 0.05 M acetate buffer containing 10 μM HBT, T = 35°C].
![Figure 2. Laccase activity optimization [Laccase activities: –•–•–: 4 U, –▪–▪–: 8 U, –▴–▴–: 2 U. Working conditions: Amount of gelatin and percentage of glutaraldehyde were kept constant as 10 mg and 2.5%, respectively. Working buffer: pH 4.5, 0.05 M acetate buffer containing 10 μM HBT, T = 35°C].](/cms/asset/afbcfe6b-9f90-498f-9273-ff8600b43f18/ianb19_a_478199_f0002_b.gif)
The surface area of the type I biosensor was about 50-fold higher than that of the type II biosensor. Consequently, the gelatin ratio in fact was the same with each biosensor. Such that, when we used laccase enzyme with 8 U, the biosensor response was 26% lower than that of the enzyme with 2 U Also, because of the above-mentioned reason, laccase activities in the biosensors were just about the same. When it comes to response times of both biosensors, there was no difference in response times. Moreover, because the type I biosensor was more sensitive than the other one, its response time was shorter than the type II by about 2 minutes. It was the result expected, because in the type I biosensor the results were directly obtained by laccase enzymatic reaction, which was monitored by oxygen decrease. However, in the second system, the biosensor was based on a cycle of mediator. Consequently, this oxidation of mediator cycle extended the biosensor response compared with the type I biosensor. Finally, the standard deviation of the measurements for 0.015 µM 2,4-D was ±0.0007 µM. As we mentioned earlier, the results showed that enzyme activity slightly affected the biosensor response.
pH dependence of the biosensors. For this purpose, the effect of pH on the type I biosensor was evaluated. Maximum biosensor response for 2,4-D was obtained at pH 4.5 (50 mM, acetate buffer solution). The response decreased above pH 4.5. In addition, below pH 4.5 at more acidic pH, biosensor response was just about 50% of its initial activity (). Consequently, optimum pH for the laccase biosensor based on the dissolved oxygen probe was decided to be pH 4.5 acetate buffer (). Hereafter, all measurements carried out with the type I biosensor were done using acetate buffer solution pH 4.5 50 mM. In addition, pH dependence of the type II biosensor was also investigated. Results showed that the effect of pH on the type II biosensor was the same as that on the type I biosensor. The best results (in the sense of biosensor signals) for both biosensors were obtained in pH 4.5, 50 mM acetate buffer.
Figure 3. pH dependence of the biosensors [–▪–▪–: oxygen electrode, –•–•–: screen printed graphite electrode. Working conditions: For type I biosensor: 2,4-D concentration used was 0.1 μM, amounts of laccase activity, gelatin and percentage of glutaraldehyde were kept constant as 4 U, 10 mg and 2.5%, respectively. For type II biosensor: 2,4-D concentration we used 1 μM; amounts of laccase activity gelatin, and percentage of glutaraldehyde were kept constant as 4 U, 0.2 mg, and 2.5%, respectively. All buffers were 0.05 M and acetate buffer solutions containing 10 μM HBT with different pHs, T = 35°C, for type II biosensor; working potential = −0.7 V].
![Figure 3. pH dependence of the biosensors [–▪–▪–: oxygen electrode, –•–•–: screen printed graphite electrode. Working conditions: For type I biosensor: 2,4-D concentration used was 0.1 μM, amounts of laccase activity, gelatin and percentage of glutaraldehyde were kept constant as 4 U, 10 mg and 2.5%, respectively. For type II biosensor: 2,4-D concentration we used 1 μM; amounts of laccase activity gelatin, and percentage of glutaraldehyde were kept constant as 4 U, 0.2 mg, and 2.5%, respectively. All buffers were 0.05 M and acetate buffer solutions containing 10 μM HBT with different pHs, T = 35°C, for type II biosensor; working potential = −0.7 V].](/cms/asset/a37377f2-27ad-481a-a664-e11254f46fe1/ianb19_a_478199_f0003_b.gif)
Our results agreed with the literature. In the articles presented on laccase biosensors, the optimum pH of the system was around 4–6 [Citation35–38]. Although the soluble laccase enzyme has an optimum pH around 3–4, the immobilization shifted the useful pH range to 4–5. In addition to the fact that the type I biosensor was slightly affected by pH changes, the performance of the type II biosensor was importantly affected by pH changes. This was probably related to immobilization procedures and the electrochemical measurement system. It is expected that the electrochemical system based on a mediator should be more sensitive to pH changes. Standard deviations of pH values 4 and 5 were 27.5 (biosensor response, %) and 7.1 (biosensor response, %), respectively.
Temperature dependence of the biosensors. The effect of temperature on the biosensors was also evaluated. First, optimum temperature of the type I biosensor was determined. A large effect of temperature on the biosensor was reported. This effect is seen in . The highest biosensor response was obtained at 35°C. The electroanalytical performance of the biosensor was increased with the increase of temperature. However, at high temperatures it is clear that the thermal denaturation of proteins is substantially occurred. For instance, at 45°C the biosensor signals dramatically decreased because of the effect mentioned above. However, in the range of 30–42°C the performance of the type I biosensor was almost the same, such that the standard deviation for 30 and 42°C was 2.8% (activity, %). Optimum temperature for the type I biosensor was accepted as 35°C.
Figure 4. Optimum temperature of the biosensors [–▪–▪–: oxygen electrode, –♦–♦–: screen printed graphite electrode. Working conditions: For type I biosensor: 2,4-D concentration we used 0.1 μM; amounts of laccase activity, gelatin, and percentage of glutaraldehyde were kept constant as 4 U, 10 mg, and 2.5%, respectively. For type II biosensor: 2,4-D concentration we used 1 μM; amounts of laccase activity, gelatin, and percentage of glutaraldehyde were kept constant as 4 U, 0.2 mg, and 2.5%, respectively. 0.05 M acetate buffer containing 10 μM HBT pH 4.5, for type II biosensor; working potential = −0.7 V].
![Figure 4. Optimum temperature of the biosensors [–▪–▪–: oxygen electrode, –♦–♦–: screen printed graphite electrode. Working conditions: For type I biosensor: 2,4-D concentration we used 0.1 μM; amounts of laccase activity, gelatin, and percentage of glutaraldehyde were kept constant as 4 U, 10 mg, and 2.5%, respectively. For type II biosensor: 2,4-D concentration we used 1 μM; amounts of laccase activity, gelatin, and percentage of glutaraldehyde were kept constant as 4 U, 0.2 mg, and 2.5%, respectively. 0.05 M acetate buffer containing 10 μM HBT pH 4.5, for type II biosensor; working potential = −0.7 V].](/cms/asset/e39e45a4-a137-45f0-a037-c2063fa20a37/ianb19_a_478199_f0004_b.gif)
Next, the temperature dependence of the type II biosensor was also evaluated. These studies showed that the temperature particularly affected the type II biosensor. There were dramatic increases and decreases in the biosensor signals with temperature changes. Although the highest response was obtained at 35°C below and above of this temperature, there was a dramatical activity decrease. These serious activity changes were probably caused by the lack of enzyme activity in the bioactive layer of the type II biosensor. At higher temperatures than 35°C, a little enzyme denaturation caused major activity loss of the biosensor since the biosensor had a little amount of the enzyme in its bioactive layer. The standard deviation for 30 and 42°C is 11.3% (activity, %). Consequently, 35°C was also the optimum temperature for the type II biosensor. In addition to working temperature, one of the most important parameters was storage temperature. When not in use the biosensors were stored at +4°C in moisture medium. Our studies showed that this temperature was useful for our biosensors. The activity loss related to storage period occurred by physical disorders of the bioactive layers of both biosensors, not the enzyme denaturation correlated storage period.
Thermal stabilities of the biosensors. Thermal stabilities of the biosensors were evaluated by incubating the biosensors at 35°C. No significant difference was noted during the 5-hour incubation period. At the end of the 8th hour, the activity of type I biosensor decreased 5% of its initial activity. The response time of the biosensor was 10 minutes. The results showed that the biosensor, type I, had a very good thermal stability. In addition, there was a decrease in type II biosensor response about 10% of its initial activity at the end of 8 hours incubation period. Its response time was 4 minutes. Its response time was shorter that that of type I. It was probably caused by filling the active centers of laccase rapidly since there was less enzyme than type II in the bioactive layer of the biosensor. Despite this, the thermal stability of type II biosensor was quite good.
Substrate Specificities
Biosensor activities were determined against a variety of compounds such as 2,4-D, phenol, catechol, pyrogallol, 2-chlorophenol, 4-nitrophenol. The results for the biosensors are summarized in . It can be seen that among all the substrates analyzed 2,4-D and phenol gave the best response for both biosensors. Moreover 2,4-D, phenol, catechol, pyrogallol, 2-chlorophenol, 4-nitrophenol showed good correlation coefficients for both biosensors.
Table 1. Biosensors responses for some phenolic compounds
As can be seen in , 2,4-D can be detected in the range of nM concentration by the type I biosensor. It was a very good result for the biosensor. Because of laccase's broad substrate specificity the biosensor showed activity against the other phenolic substrates. When it comes to comparison of the biosensors, the results showed that the specificity of the type I biosensor was higher than the type II biosensor. For 2,4-D the type I biosensor had a better lowest detection limit such as 5 nM. However, the other biosensor had a lowest detection limit of 500 nM for 2,4-D. A similar effect was observed for the other substrates tested. All of the substrates were analyzed with lower detection limits by the type I biosensor than the type II biosensor. This could be related with the amount of enzyme activity immobilized in the bioactive layer. The type II biosensor contained relatively little enzyme. Because, at relatively high temperatures the secondary chemical interactions such as ionic, dipole-dipole, and hydrofobic interactions or hydrogen bonds should be disrupted. Since these forces make possible stability and catalytic activity of the enzymes, they mostly lost of their natural activities at high temperatures. Consequently, the activity of the biosensor or the detection limits of the phenolic compounds studied were not as good as the type I biosensor. Moreover, the other factor that could affect the performance of the type II biosensor was diffusion effect. The brown-colored polymer was deposited on the surface of the bioactive layer and this product resulted in an additional diffusion barrier. Due to the fact of these two limitations, the type II biosensor showed slightly lower activity to the phenolic compounds than that of the type I biosensor.
Analytical Characteristics of the Biosensors
Linear ranges. shows the calibration curve of the type I biosensor for 2,4-D. A very good linear relationship with a correlation coefficient of 0.9948 was obtained over the concentration range from 5.10−9 M- 2.10−8 M 2,4-D. The minimum detectable concentration of 2,4-D was estimated to be 5.10−9 M. For the type II biosensor a calibration curve is shown in . The lowest 2,4-D concentration measurable with the biosensor was 25.10−8 M. For the values plotted in the correlation coefficient was 0.9933. The linear concentration range was 25.10−8 M – 2.10−6 M.
Figure 5. Calibration curves of Type I (a) and Type II (b) biosensors for 2,4-D [Working conditions for Type I biosensor: Amounts of laccase activity, gelatin, and percentage of glutaraldehyde were kept constant as 4 U, 10 mg, and 2.5%, respectively. 0.05 M pH 4.5 acetate buffer containing 10 μM HBT, T = 35°C. Working conditions for Type II biosensor: Amounts of laccase activity, gelatin, and percentage of glutaraldehyde were kept constant as 4 U, 0.2 mg, and 2.5%, respectively. 0.05 M pH 4.5 acetate buffer containing 10 μM HBT, T = 35°C, working potential = −0.7 V].
![Figure 5. Calibration curves of Type I (a) and Type II (b) biosensors for 2,4-D [Working conditions for Type I biosensor: Amounts of laccase activity, gelatin, and percentage of glutaraldehyde were kept constant as 4 U, 10 mg, and 2.5%, respectively. 0.05 M pH 4.5 acetate buffer containing 10 μM HBT, T = 35°C. Working conditions for Type II biosensor: Amounts of laccase activity, gelatin, and percentage of glutaraldehyde were kept constant as 4 U, 0.2 mg, and 2.5%, respectively. 0.05 M pH 4.5 acetate buffer containing 10 μM HBT, T = 35°C, working potential = −0.7 V].](/cms/asset/228242a8-2f06-4c49-ad47-506277844157/ianb19_a_478199_f0005_b.gif)
Accuracy. The repeatability studies were also carried out for both biosensors. The repeatability of the type I biosensor was studied for 0.01 μM 2,4-D standard concentration (n=5). The standard deviation and variation coefficient were calculated as ±0.0003 μM and 2.7%, respectively. Moreover, accuracy for the type II biosensor was tested for 0.5 μM 2,4-D (n=5). The standard deviation and variation coefficient were calculated as ±0.014 μM and 4.66%, respectively.
Sample analysis. This part of the study included the application of developed biosensors for determination of 2,4-D in herbicide samples. shows these results with standard addition method for both biosensors. In these experiments herbicide samples with appropriate dilutions were added to the reaction cell.
Table 2. Real sample analyses with the biosensors proposed
Experimental results indicated that good recovery was observed. In addition, good agreement between the results of the biosensor presented was achieved. When a comparison is made between the S.D. values of the biosensors it can be shown that the type I biosensor has a better S.D value than that of the type II biosensor. In fact, these results also could be correlated with the limitations mentioned in the section of substrate specifics. These results indicate that the proposed biosensors can be applied successfully for the determination of 2,4-D.
CONCLUSION
We have described two biosensors for determination of 2,4-D in herbicide samples. From a comparison of analytical performances of both types of biosensors it was apparent that the better performance was of the type I biosensor. This result probably was caused by the sensitivity and specificity of oxygen probes. Very little changes in the dissolved oxygen concentrations could be detected by the oxygen probe and oxygen meters. Consequently, the type I biosensor showed better performance than the biosensor mediated by hydroxy benzotriazole. However, by using the second system, type II biosensor, the phenolic compounds were also successfully detected. Moreover, the type II biosensor contained a little enzyme, and immobilization materials, gelatin and glutaraldehyde. Consequently, the type II biosensor was extremely economic. The biosensors showed good activities towards the same kind of phenolic compounds. The proposed laccase biosensors exhibited wide linear detection range, acceptable reproducibility, and thermal stability. The detection limits of the biosensors for 2,4-D were very good. 2,4-D could be detected by the type I biosensor in the range of nM concentration. In addition, with the type II biosensor good results as the same as type I biosensor couldn't be obtained. Because we obtained a very good concentration range from 5.10−9 M – 2.10−8 M for 2,4-D by the type I biosensor. Beside the minimum detectable concentration of 2,4-D was estimated to be 5.10−9 M for type I biosensor. However, linear concentration range obtained by type II biosensor was 5.0−5 M – 2.10−6 M. And the detection limit of the type II biosensor was 5.10−6 M. However, if we compare between the type II biosensor and similar biosensor systems in the literature, our biosensor system, type II, exhibits better characteristics than the studies in the literature [Citation35, Citation38]. The same results appeared when worked with the other phenolic compounds. These phenolic compounds were detected by a type II biosensor with lower sensitivity. The sensitivity of the type II biosensor may be improved by using extra pure laccase enzyme. In addition, the mediator system should be changed. Instead of hydroxy benzotriazole, another mediator that could be more effectively diffused to the electrode surface from bulk solution should be used. In terms of the lowest detection limits of the biosensors, the type I biosensor always showed the best results. The applicability of both biosensors to the real herbicide samples was also very good. Finally, the most important limitations of two biosensor systems were group specifications of laccase enzyme. Because of the group specificity of the laccase enzyme, the biosensors showed activity towards the other phenolic compounds with different magnitudes. Nevertheless, this limitation has occurred when the laccase enzyme was used for the other biosensor systems in the literature.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Odaci, D., Timur, S., Pazarlioglu, N., Montereali, M.R., Vastarella, W., Pilloton, R., Telefoncu, A. (2007). Determination of phenolic acids using Trametes versicolor laccase. Talanta 71(1): 312–317.
- Peter, M.G. and Wollenberger, U. (1997). Phenol-oxidizing enzymes: mechanisms and applications in biosensors. EXS 80: 63–82.
- Amitai, G., Adani, R., Sod-Moriah, G., Rabinovitz, I., Vincze, A., Leader, H., Chefetz, B., Leibovitz-Persky, L., Friesem, D., Hadar, Y. (1998). Oxidative biodegradation of phosphorothiolates by fungal laccase. FEBS Lett. 438: 195–200.
- Mougin, C., Kollmann, A., Jolivalt, C. (2002). Enhanced production of laccase in the fungus Trametes versicolor by the addition of xenobiotics. Biotechnol. Lett. 24: 39–42.
- Abadulla, E., Tzanov, T., Costa, S., Robra, K.H., Cavaco-Paulo, A., Gubitz, G.M. (2000). Decolorization and detoxification of textile dyes with a laccase from Trametes hirsuta. Appl. Environ. Microbiol. 66: 3357–3362.
- Mayer, A.M. and Staples, R.C. (2002). Laccase: new functions for an old enzyme. Phytochemistry 60: 551–565.
- Thurston, C.F. (1994). The structure and function of fungal laccases. Microbiology 140: 19–26.
- Bourbonnais, R., Paice, M.G. (1990). Oxidation of non-phenolic substrates. An expanded role for laccase in lignin biodegradation. FEBS Lett.267(1): 99–102.
- Bolobova, A.V., Askadsky, A.A., Kondrashenko, V.I., Rabinovich, M.L. (2002) . Theoretical Bases of Biotechnology of Wood Aggregates. Part II: Enzymes, Models, Processes, Science, Moscow.
- Rochefort, D., Bourbonnais, R., Leech, D., Paice, M.G. (2002). Oxidation of lignin model compounds by organic and transition metal-based electron transfer mediators. Chem. Commun. 11: 1182–1183.
- Shleev, S.V., Khan, I.G., Gazaryan, I.G., Morozova, O.V., Yaropolov, A.I. (2003). Novel laccase redox mediators: spectral, electrochemical, and kinetic properties. Appl. Biochem. Biotechnol. 111: 167–184.
- Rochefort, D., Leech, D., Bourbonnais, R. (2004). Electron transfer mediator systems for bleaching of paper pulp. Green Chem. 6: 14–24.
- Odaci, D., Timur, S., Pazarlioğlu, N., Kirgoz U.A., Telefoncu, A. (2006). The effect of mediator on laccase biosensor response for paracetamol detection. Biotechnol. Appl. Biochem. 45: 23–28.
- Shleev, S., Persson, P., Shumakovich, G., Mazhugo, Y., Yaropolov, A., Ruzgas, T., Gorton, L. (2006). Interaction of fungal laccases and laccase-mediator systems with lignin. Enzyme Microb. Techn. 39: 841–847.
- Torres, E., Bustos-Jaimes, I., Borgne, S.L. (2003). Potential use of oxidative enzymes for the detoxification of organic pollutants. Appl. Catal. B-Environ. 46: 1–15.
- Best, G.A. (1995). Turhven, A.D., ed., Pesticides-Developments, Impacts, and Control, Royal Society of Chemistry, Cambridge.
- World Health Organization. (1989). 2,4-Dichlorophenoxyacetic acid (2,4-D)-environmental aspects. Environmental Health Criteria 84. International Programme on Chemical Safety, WHO, Geneva.
- Gorzinski, S.J., Kociba, R.J., Campbell, R.A., Smith, F.A., Nolan, R.J., Eisenbrandt, D.L. (1987). Acute pharmacokinetic, and subchronic toxicological studies of 2,4-dichlorophenoxyacetic acid. Fundam. Appl. Toxicol. 9: 423–435.
- Moody, R.P., Franklin, C.A., Ritter, L. and Maibach, H.I. (1990). Dermal absorption of the phenoxy herbicides 2,4-D, 2,4-D amine, 2,4-D isooctyl, and 2,4,5-T in rabbits, r.t., rhesus monkeys, and humans: a cross-species comparision. J. Toxicol. Environ. Health. 29(3): 237–245.
- Kaioumova, D., Susal, C., Opelz, G. (2001). Induction of apoptosis in human lymphocytes by the herbicide 2,4-dichlorophenoxyacetic acid. Hum. Immunol. 62: 64–67.
- Tuschl, H., Schwab, C. (2003). Cytoxic effects of the herbicide 2,4-dichlorophenoxyacetic acid in HepG2 cells. Food Chem. Toxicol. 41: 385–393.
- Ibrahim, M.A., Bond, G.G., Burke, T.A., Cole, P. Dost, F.N., Enterline, P.E., Gough, M., Greenberg, R.S., Halperin, W.E., McDonnel, E. (1991). Weight of the evidence on the human carcinogenicity of 2,4-D. Environ. Health Perspect. 96: 213–222.
- Uesugi, Y., Ueji, M., Koshioka, M. (1997) . Pesticide Data Book, 3rd ed., Soft Science Publications, Tokyo.
- Anastassiades, M., Lehotay, S.J., Stajhbaher, D., Schenck, F.J. (2003). Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 86: 412–431.
- Ding, W.H., Liu, C.H., Yeh, S.P. (2000). Analysis of chlorophenoxy acid herbicides in water by large-volume on-line derivatization and gas chromatography-mass spectrometry. J. Chromatogr. A. 896: 111–116.
- Nilsson, T., Baglio, D., Galdo-Miguez, I., Ogaard-Madsen, J., Facchetti, S. (2002). Derivatisation/solid-phase microextraction followed by gas chromatography–mass spectrometry for the analysis of phenoxy acid herbicides in aqueous samples. J. Chromatogr. A. 826: 211–216.
- King, J.W., Zhang, Z.Y. (2002). Derivatization reactions of carbamate pesticides in supercritical carbon dioxide. Anal. Bioanal. Chem. 374: 88–92.
- Dicklow, L.M., Gerken, D.F., Sams, R.A., Ashcraft, S.M. (2001). Simultaneous determination of 2,4-D and MCPA in canine plasma and urine by HPLC with fluorescence detection using 9-Anthryldiazomethane (ADAM). J. Anal. Toxicol. 25: 35–39.
- Franek, M., Kolfir, V., Granitovi, M. and Nevorinkovi, Z. (1994). Monoclonai ELISA for 2,4-dichlorophenoxyacetic acid. Characterisation of antibodies and assay optimisation. J. Agric. Food Chem. 42: 1369–1374.
- Meulenberg, E.P. and Stoks, P.G. (1995). Water quality control in the production of drinking water from river water. The application of immunological techniques for the detection of chlorophenoxy acid herbicides (2,4-D). Anal. Chim. Acta. 311: 407–413.
- Matuszczyk, G., Knopp, D. and Niebner, R. (1996). Development of an ELISA for 2,4-D: characterisation of two polyclonal antisera. Fresenius J. Anal. Chem. 354: 41–47.
- Kalab, T. and Skladal, P. (1995). A disposable amperometric immunosensor for 2,4-dichlorophenoxyacatic acid. Anal. Chim. Acta. 304: 361–368.
- Skladal, P., Kalab, T. (1995). A multichannel immunochemical sensor for the determination of 2,4-dichlorophenoxyacetic acid. Anal. Chim. Acta. 316: 73–78.
- Wittmann, C., Bier, F.F., Eremin, S.A. and Schmid, R.D. (1996). Quantitative analysis of 2,4-dichlorophenoxyacetic acid in water samples by two immunosensing methods. J. Agric. Food Chem. 44: 343–350.
- Jegan Roy, J., Emilia Abraham, T., Abhijith, K.S., Sujith Kumar, P.V., Thakur, M.S. (2005). Biosensor for the determination of phenols based on Cross-Linked Enzyme Crystals (CLEC) of laccase. Biosens. Bioelectron. 21: 206–211.
- Fernandes, S.C., de Oliveira, I.R., Fatibello-Filho, O., Spinelli, A., Vieira, I.C. (2008). Biosensor based on laccase immobilized on microspheres of chitosan crosslinked with tripolyphosphate. Sen. Act. B Chem. 133: 202–207.
- Vianello, F., Ragusa, S., Cambria, M.T., Rigo, A.. (2006). A high sensitivity amperometric biosensor using laccase as biorecognition element. Biosens. Bioelectron. 21: 2155–2160.
- Rochefort, D., Kouisni, L., Gendron, K. (2008). Physical immobilization of laccase on an electrode by means of poly(ethyleneimine) microcapsules. J. Electroanal. Chem. 617: 53–63.