Abstract
The whole blood immuno-adsorption (WBIA) system, using an adsorbent to remove pathogenic antibodies of Myasthenia Gravis (MG), was studied. Cellulose-tryptophan adsorbent was synthesized and purified in our lab. Experimental autoimmune myasthenia gravis (EAMG) rabbits were passively transferred with immunoglobulin from patients with myasthenia gravis. The rabbits underwent extracorporeal whole blood adsorption for 2 hours. Results showed no significant damage to blood cells and no changes in the concentrations of electrolytes. Total protein decreased by 12.6% (P<0.05) and globulin protein decreased 21.9% (P<0.05). The overall removal of antibodies against nicotinic acetylcholine receptor (nAChR) was 49.85%. The percentage of decrement of compound muscle action potential in 3, 5, 10Hz of EAMG rabbits all dropped down after the treatment. The quantity of neuromuscular junctions per unit area (25 mm2) increased significantly after treatment (P<0.05). In conclusion, the adsorbent was biocompatible, safe for whole blood immuno-adsorption. Whole blood immuno-adsorption improved clinical manifestation and neuromuscular function of the passively transferred EAMG rabbits.
INTRODUCTION
Myasthenia gravis (MG) is an autoimmune disease characterized by the weakness of striated muscles. The weakness is due to impaired neuromuscular transmission, resulting from a reduction of receptors for neurotransmitter acetylcholine (ACh) at the post-synaptic neuromuscular junction (NMJ). The damage is attributed to the auto-antibodies against nicotinic acetylcholine receptors (AChR) on the skeletal muscles. Anti-AChR antibodies (AChR-ab) can cause loss of receptors, or directly inhibit their function [Citation1]. Experimental autoimmune myasthenia gravis (EAMG) is a perfect animal prototype of myasthenia gravis. The latter can be passively transferred with anti-acetylcholine receptor antibodies from patients of myasthenia gravis or EAMG animals [Citation2–5]. Anti-AChR antibodies belong to the IgG subclass, therefore plasma exchange (PE), double filtration plasma-pheresis (DFPP), and immuno-adsorption plasma-pheresis (IAPP) have been used for the treatment of MG in order to remove anti-AchR antibodies. However, PE and DFPP are limited by their non- or semi-selective removal of plasma components. IAPP is superior to PE and DFPP because the adsorbent columns used could selectively remove specific pathogenic substances, but cost-effectiveness and disease infection limited its use.
It has been reported that AchR-ab could be removed by an absorbent linked with tryptophan, which was used to treat 20 MG patients by Shibuya et al. in 1992 [Citation6] and 16 MG patients by Grob et al. in 1995 [Citation7]. Yan WR et al. [Citation8] have studied the adsorption capacities of various ligands for the removal of pathogenic antibodies in MG in vitro and found that immuno-adsorbent prepared by coupling L-tryptophan to epichlorohydrin-activated cellulose bead showed the best result. We have used this absorbent to treat experimental autoimmune myasthenia gravis, induced by Torpedo California, Ta183–200, in extracorporeal (WBIA) and improved the clinical symptoms significantly [Citation9].
In the present paper, we developed a whole blood immuno-adsorption system as a treatment modality for EAMG and studied the therapeutic efficacy and safety of the system, with cellulose-tryptophan adsorbent, for the treatment of passive experimental myasthenia gravis.
MATERIALS AND METHODS
IgG of MG Patients
Eighty patients, with typical myasthenia gravis (47 females and 33 males from 11-63 years of age) and anti-AChR antibodies sero-positive, were informed with consent. A total of 2400 ml blood was collected from the 80 patients. In each case, the diagnosis was established by the typical history and physical findings of weakness and fatigue. The diagnosis was confirmed by decrement electrical responses of muscles to repetitive nerve stimulation and by improvement of muscle strength with anti-cholinesterase medications. All the patients were receiving myasthenia, but none had prior thymectomy or adrenal corticosteroid medication.
The blood was allowed to clot, and the serum was removed after centrifugation and stored at −70°C until further procession. Crude immunoglobulin fractions were precipitated from pooled sera by adding 33% saturated ammonium sulphate. Crude immunoglobulin was purified by chromatography on DEAE-Sephadex A-50 columns. The total amount of purified immunoglobulin G(Ig G) containing AChR-Ab of MG patients was stored at −70°C.
Induction of Experimental Autoimmune Myasthenia Gravis Animal Models
Sixteen female rabbits weighing approximately 2Kg were injected with AChR-Ab. Each rabbit received daily intraperitoneal injections of 1 ml of Ig G for 3 days. Twenty-four hours after the first injection, each rabbit was given cyclophosphamide (300mg/Kg) intra-peritoneal injections in order to induce tolerance to the human serum proteins. WBIA was performed on the eight female rabbits without injection.
Clinical Assessment of MG in Animal Model
The passively transferred rabbits were examined daily for clinical signs of EAMG. After the second day of the passive injection, all the rabbits showed clinical signs of fatigue and weakness. The weakness exacerbated on the third day. Clinical symptoms could be improved by neostigmine.
On the day before and the 5th day after the passive transfer, the disease was graded as [Citation10]: 0, not abnormal; +, mild (weight loss and ear weakness); ++, moderate (generalized weakness and difficulty in walking); +++, severe (flaccid quadriplegia, severe weight loss, and respiratory insufficiency).
EAMG rabbits were successfully induced by passively transferred IgG of myasthenia gravis patients. According to the grade of clinical symptoms 16 EAMG rabbits were divided into a therapeutic group and a control group. The rabbits in the therapeutic group underwent extracorporeal whole blood adsorption while the control group was not perfused.
Preparation of Adsorbents
Cellulose beads as the matrix were synthesized according to the method of De-Ling Kong (Citation11). The beads were activated by epichlorohydrin and tryptophan was coupled on the beads as a ligand.
Assay of Serum Anti-AChR Antibodies
The antibody against AChR was determined by indirect enzyme linked immuno-sorbent assay (ELISA). Briefly, 96-well flat-bottomed plates were coated with 0.4ug/ml ofα-bungarotoxin (α-BTX) in PBS overnight at 4°C. The plates were then washed with PBS and incubated with serum samples overnight at 4°C. Then the plates were incubated with horseradish peroxidase labeled with goat Ab against human rabbit immuno-globulins at 37°C for 2hr. After washing with PBS, p-nitrophenyl phosphate was added, and the optical density (OD) at 492nm was recorded as the titers of antibody.
Assessment of Electro-physiological Function
Repetitive nerve stimulation (RNS) was performed under sodium pentobarbital anesthesia using a conventional clinical electromyography apparatus (Counterpoint MK2, DANTEC). The compound muscle action potential of the deep peroneal nerve was recorded in response to stimuli at 3, 5, 10Hz, The decrement was evaluated by comparing the amplitude of the fifth to the first muscle action potential.
Measurement of Blood Components
Blood cell counts, concentration of proteins, and electrolytes in plasma were all auto-analyzed by conventional methods (Automatic Analyzer, 7170A HITACHI and Blood Cell Analyzer, XE-2100 SYSMEX).
Determination of the Adsorption Capacity for AchR-ab
1 ml cellulose-tryptophan beads was incubated with 3.0ml AChR positive serum and stirred for 3 hours at 37°C. AChR-ab titers were determined by ELISA. The adsorption capacity was calculated as the percentage decrease of the plasma OD at 492 nm.
Quantity of Neuromuscular Junctions
Calf muscle specimens from the rabbits and the fresh tissues were quickly frozen and sectioned into 5-μm. Neuromuscular junctions were counted by immuno-histochemistry. 1:50 diluted horseradish peroxidase-labeledα-BTX (HRP-α-BTX) was added and reacted for 2h at 37°C. After washing with PBS, diaminobenzidine (DAB)was added to form the color reaction.
Whole blood Extracorporeal Immuno-adsorption
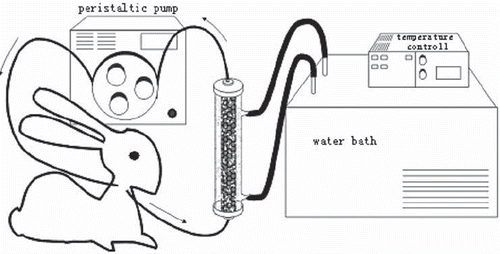
The WBIA circuit is shown in . The column was packed with 3g cellulose-tryptophan adsorbents. The beads were retained by 40 mesh filter fitted at the bottom of the column. The complete system was equilibrated with sterile heparinized saline (3μ/ml) before use. Blood was pumped from the carotid arterial catheter through an adsorbent column at a flow rate of 10-15ml/min and then returned back via the cannula in the jugular vein. The rabbits were fully heparinzed by injection of heparin and maintained in an anti-coagulated state by discrete addition as needed according to clotting times determined during the experiment. The procedure was carried out for 2 hrs and the extracorporeal blood was maintained in a water bath at a constant temperature of 39.4°C.
Experimental Setup
WBIA was performed with 8 healthy rabbits and 8 EAMG rabbits in the therapeutic group. Blood samples from the healthy rabbits were withdrawn before and after the procedure for test of elements and soluble components in plasma.
Blood was withdrawn from EAMG rabbits in the therapeutic group at present intervals for assessment of the titers of anti-AchR antibody in serum. Before perfusion and on the fifth day the EAMG rabbits were graded. Electro-physiological functions and neuromuscular junctions were assayed. Student t test was used for statistic analysis.
On the fifth day (D5) after passive transfer, extracorporeal WBIA was conducted on the rabbits of the therapeutic group. The blood circuit was kept passing through the cellulose-tryptophan column for 120 minutes, immuno-adsorption capability was measured, and the improvement of clinical manifestation was evaluated by clinical score, antibody titers, neuromuscular transmission function and neuromuscular junction tests . Student t test was used for statistic analysis.
RESULTS
Biocompatibility of the Adsorbent
Changes in blood components from a 2hr-course are shown in . No significant change of blood cell counts, haematocrits, haemoglobin, and plasma electrolytes (p>0.05) was observed. Although there was no significant decrease in albumin, the immuno-adsorption caused a noticeable decrease in total protein concentration, due to IgG removal.
Table 1. Changes in blood components after 2 hrs WBIA
Changes of Antibodies in Rabbits of the Therapeutic Group after WBIA
Immuno-adsorbent with cellulose as the carrier and tryptophan as the ligand was used to adsorb the antibodies in EAMG effectively. From we can see that a large amount of antibodies was removed during the first 60 minutes, which was 39.86±5.86% and immuno-adsorption reached a maximum value from 90 to 120 minutes. The overall removal of antibodies was 49.85±2.55% within 2 hours. From , the OD value at 492 nm of antibodies decreased significantly from 0.5389±0.1077 to 0.2690±0.0481 (P<0.001 On the 3rd day (D8) after immuno-adsorption, the OD value (0.2631± 0.0432) was significantly lower than that of the control group (0.5226 ±0.1012) on the 8th day (D8) after passive transfer (P<0.01).
Table 2. Changes of antibodies removal rate in rabbits of the therapeutic group after 2 hrs WBIA
Table 3. Changes of blood antibodies OD value in rabbits of the therapeutic group after 2 hrs (492 nm) WBIA
Assessment of Neuromuscular Transmission Function
On the 5th day after passive transfer (D5) and the 3rd day after WBIA (D8), stimulation of the deep peroneal nerve showed that the mean decrement of potentials evoked from the anterior tibial muscle at three stimulation frequencies in therapeutic group rabbits. At 3Hz the potential decreased from 21.87% to 17.87%, at 5Hz decreased from 22.25% to 18.75%, and at 10 Hz decreased from 24.37% to 23.25%. showed the changes of the electro-physiological features of EAMG rabbits after WBIA. On the 5th (D5) and 8th (D8) day after passive transfer (D8), the same RNS was performed in the control group rabbits, but no decrement was found.
Table 4. Comparison of the decrement of RNS between the therapeutic group and the control group
Therapeutic Effect on EAMG by WBIA Assessment of Clinical Manifestation
All of the EAMG rabbits in the therapeutic group after therapy had significant improvement in strength, starting from 24-48 hrs of WBIA treatment, and continued to improve during the perfusion. At the same time the EAMG rabbits in the control group showed no improvements (). On the 3rd day (D8) after WBIA (i.e., the 8th days after passive transfer), the therapeutic group rabbits underwent 4 grading levels of clinical manifestation, and compared with that on the day before WBIA (D5).
Table 5. Comparison of grading of clinical manifestation on the 5th day (D5) and 8th day (D8) after passive transfer between the therapeutic and the control group
Quantity of Neuromuscular Junction Assay
On the 3rd day after WBIA (D8), the quantity of neuromuscular junctions per unit area (25mm2) of the therapeutic group rabbits increased from 9.825±3.401 to 10.90±2.879 (P<0.05), which was significantly higher than that of the control group (P<0.01) ( and ).
DISCUSSIONS
Pinching [Citation12] has reported the remission of myasthenia gravis after plasma exchange, which was due to the removal of auto-antibodies of myasthenia gravis. Anti-AChR antibodies belong to the IgG subclass; therefore plasma exchange, double filtration plasmapheresis, and plasma-perfusion have been used for the treatment of MG in order to remove anti-AChR antibodies. However, these methods have the disadvantage that loss of useful plasma components is inevitable and lack of specific removal of antibodies. Therefore specific adsorption columns or adsorbents such as Medisorba MG-50, tryptophan-linked polyvinyl alcohol gel (IM-TR), and Ig-ADSOPAK were developed and are effective in improving the symptoms of myasthenia [Citation13,Citation6,Citation14]. This specific extracorporeal immuno-adsorption (ECIA) removed the circulating antibodies from plasma. However, there are some drawbacks to using a plasma ECIA system, such as hypervolemic effects, hemolysis during plasma separation, the complexity of tubing connection,s and the length of time required for preparation. Therefore we developed a new method of WBIA system for removal of the autoantibodies of EAMG rabbits induced by Ta183-200 and improved the symptoms effectively [Citation9].
The adsorbent with cellulose as carrier and tryptophan as ligand had good biocompatibility properties in extracorporeal whole blood immuno-adsorption. In our in-vivo trials, the effect of adsorbents on blood cells was satisfactory. There was no significant reduction on WBC, RBC, and Plt after perfusion (p>0.05). The soluble blood components, K+, Na+, Cl−, were unaffected by the WBIA system (P>0.05). Likewise, albumin concentration remained constant (p>0.05). The total protein decrease was mainly due to the loss of globulin proteins (p<0.05). The reduction of globuline protein indicated that tryptophan as a ligand could effectively remove the AChR antibody by hydrophobic binding force [Citation15]. Although the globuline protein decreased significantly (p<0.01), there was no need for substitute fluids.
After 2hr WBIA, the removal of Ab was effective and the neurotransmission function of EAMG rabbits improved. The decrement of CAMP reduced, and the pronounced and rapid recovery from MG weakness was noted in almost all of the EAMG rabbits. The quantity of neuromuscular junctions also increased on the 3rd day after the therapy. Most of the control EAMG animals without treatment showed no significant improvement in clinical manifestation, antibody titers, neuromuscular transmission function, and neuromuscular junctions at the 8th day after passive transfer.
In conclusion, our system can effectively remove the AChR-Abs. Extracorporeal whole blood immuno-adsorption with cellulose-tryptohan as absorbent is effective and safe in treating passive experimental autoimmune myasthenia gravis, which improved clinical manifestation, antibody titers, neuromuscular transmission function, and neuromuscular junction in 2 hours perfusion. The WBIA therapy has a high potency of clinical applications for the future.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Thanvi, B.R., Lo, T.C. (2004). Update on myasthenia gravis. Postgrad Med J 80(950): 690–700.
- Burges, J., Vincent, A., Molenaar, P.C., . (1994). Passive transfer of seronegative myasthenia gravis to mice. Muscle Nerve 17(12): 1393–400.
- Toyka, K.V., Drachman, D.B., Pestronk, A., . (1975). Myasthenia gravis: passive transfer from man to mouse. Science 190: 397–9.
- Lindstrom, J.M., Engel, A.G., Seybold, M.E., . (1976). Pathological mechanisms in experimental autoimmune myasthenia gravis II. Passive transfer of experimental autoimmune myasthenia gravis in rats with antiacetylcholine receptor antibodies. J Exp Med 144: 739–53.
- Engel, A.G., Sakakibara, H., Sahashi, K., . (1979). Passively transferred experimental autoimmune myasthenia gravis. Sequential and quantitative study of the motor end-plate fine structure and ultrastructural localization of immune complexes (IgG and C3), and of the acetylcholine receptor. Neurology 29: 179–88.
- Shibuya, N., Sato, T., Osame, M., . (1994). Immuno-adsorption therapy for myasthenia gravis. J Neuro Neurosur Psychia 57: 578–81.
- Grob, D., Simpson, D., Mitsumoto, H., . (1995). Treatment of myasthenia gravis by immuno-adsorption of plasma. Neurology 45: 338–44.
- Yan, W.R., Yu, Y.T., Yang, L. (2002). Studies on new immuno-adsorbent for myasthenia gravis. Chemical Journal of Chinese Universities 23(10): 1887–90.
- Yang, L., Cheng, Y., Yan, W.R., . (2004). Extracorporeal whole blood immuno-adsorption of autoimmune myasthenia gravis by cellulose tryptophan adsorbent. Artif Cells Blood Substit Immobil Biotechnol 32(4): 519–28.
- Karussis, D.M., Lehmann, D., Brenner, T., . (1994). Immuno-modulation of experimental autoimmune myasthenia gravis with linomide. Journal of Neuroimmunology 55: 187–93.
- Kong, D.L., Chen, C.Z., Lin, E.F., Yu, Y.T. (1998). Clinical trials of type I and II in vitro studies of immuno-adsorbents for systemic lupus erythematosus therapy. Artificial Organs 22(8): 644–650.
- Pinching, A.J., Peters, D.K. (1976). Remission of myasthenia gravis following plasma-exchange. Lancet 2(8000): 1373–6.
- Nakaji, S., Hayashi, N. (2003). Adsorption column for myasthenia gravis treatment: Medisorba MG-50. Therap Apher Dial 7(1): 78–84.
- Ptak, J., Lochman, J. (2005). Immuno-adsorption therapy and complement activation. Transfus Apher Sci 32(3): 263–7.
- Yoshida, M., Tamura, Y., Yamada, Y., . (2000). Immusorba TR and Immusorba PH: basics of design and features of functions. Ther Apher 4(2): 127–34.