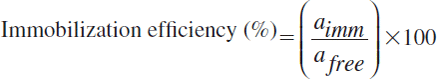Abstract
Polyphenol oxidase (PPO, EC 1.14.18.1) was isolated from artichoke head (Cynara scolymus L.) by using 0.1 M Tris-HCl buffer (pH 7.0), concentrated by (NH4)2SO4 precipitation, and immobilized in copper-alginate beads. Immobilization yield was determined to be 70%. The cresolase and catecholase activities of enzyme immobilized at optimum immobilization conditions were found to be 13.3 and 670 U g beads min−1, respectively. Effects of immobilization conditions such as alginate concentration, CaCl2 concentration, amount of loading enzyme, bead size, and amount of beads on enzymatic activity were investigated. Optimum alginate and CuCl2 concentration were found to be 2 % and 3 % (w/v), respectively. Using bead (diameter 3 mm) amount of 0.25 g maximum enzyme activities were observed for both polyphenol activities. The initial concentrations of loading free enzyme were 6.5U mL−1 and 5815 U mL−1 for cresolase activity and catecholase activities, respectively. Beads prepared at optimum immobilization conditions were suitable for up to 8 repeated uses.
INTRODUCTION
Enzymes are usually used in an environment quite different from their natural habitat. They can be inactive in most enzymatic reactions performed in aqueous media. One of the most used strategies to improve the stability of enzymes is immobilization in solid carriers [Citation1]. Immobilization of the enzyme is undertaken either for the purpose of basic research or for use in technical processes of commercial interest. The increasing use of immobilized enzymes as catalysts in various industrial processes is mainly because of their advantages. These include increased enzymatic stability in extreme conditions of temperature, pH, organic solvent, recovery, and reuse of enzymes and, in the case of proteases, removal or reduction of autodigestion.
Various techniques have been developed for enzyme immobilization, including adsorption to insoluble materials, entrapment in polymeric gels, encapsulation in membranes, cross-linking with bifunctional reagents, or covalent linking to an insoluble carrier. Of these, entrapment is one of the simplest methods of immobilization under milder conditions, and therefore results in minimum denaturation of the biocatalysts during the process [Citation2]. There are a variety of polymers available for enzyme entrapment. Alginate, naturally occurring polysaccharide that forms gels by ionotropic gelation, is the most popular of all supports.
Entrapment in calcium alginate gel is one of the simple methods of immobilization. However, there are some limitations such as low stability and high porosity of gel. These properties could lead to leakage of the enzymes. The affinity of alginate gel for the divalent cation such as Ca2+, Cu2+, Ba2+ increases the hardness and mechanical strength of the gel [Citation3]. These divalent cations lead to gelation and produce more stable gels. Because of the higher affinity of alginate for Cu2+ with respect to Ca2+, Cu2+ has been widely used for immobilization of polyphenol oxidase. Therefore, copper chloride solution was used as the cross-linking agent in this study.
Polyphenol oxidase (EC 1.14.18.1) is a copper-containing enzyme, which exhibits both a monophenol oxidase activity (cresolase activity) and a diphenol oxidase activity (catecholase activity) via separate active sites. Catecholase activity involves the removal of two hydrogen atoms from an o-diphenol such as catechol, chlorogenic acid, or 3,4-dihydroxyphenylalanine (l-DOPA) to form the corresponding o-quinone. Cresolase reactions catalyse the oxidation of monohydric phenol such as tyrosine and o-cresol to form a hydroxyl group at the ortho position. Both l-tyrosine and l-DOPA, the respective natural substrates for each of the activities, give the final product dopachrome. To produce l-DOPA from l-tyrosine, it is necessary to inhibit further oxidation to dopachrome with simultaneous ascorbate oxidation[Citation4, Citation5].
The immobilization of polyphenol oxidase from the different sources such as mushroom, potato, and frog epidermis using the various immobilization materials like copper-alginate, polyacrylamid, gelatin, Nylon 6,6, zeolite, sepiolite and glass beads has been reported by many researchers in the literature [Citation3–16].
In this study, poyphenol oxidase isolated from artichoke heads, which is a different source from the previously mentioned sources, was first immobilized within copper-alginate gel beads. Immobilization parameters such as gel concentration, CuCl2 concentrations, bead size and amounts were optimized. Also, cresolase activities and catecholase activities of artichoke polyphenol oxidase (free or immobilized) were compared from the point of view of these parameters.
EXPERIMENTAL
Materials
Sodium alginate was purchased from Fluka Biochemika; l-tyrosine, l-DOPA and l-ascorbic acid were obtained from Sigma. Catechol and CuCl2 were purchased from Merck. All the remaining reagents were of analytical grade and used without further purification.
Isolation of Polyphenoloxidase
For the preparation of the crude extracts, 100 g of artichoke head were separated, cut quickly into thin slices, and homogenised with 200 mL of 0.1 M Tris-HCl, pH 7.0 containing 10 mM ascorbic acid, 0.1% polyvinylpyrrolidone and 0.5% Triton X-100 in a blender for 3 min. The homogenate was filtered through cheesecloth, and the filtrate was centrifuged at 10000 × g for 30 min at 4°C. The supernatant was brought to 10–90% (NH4)2SO4 saturation with solid (NH4)2SO4. The precipitated PPO was separated by centrifugation at 10000 × g for 30 min at 4°C. The precipitate was dissolved in a small amount of 0.1 M Tris-HCl, pH 7.0, and dialysed at 4°C in the same buffer, changing the buffer every eight hours three times during dialysis [Citation17].
Assay of Catecholase and Cresolase Activities
Catecholase activity was determined by measuring the increase in absorbance at 420 nm for catechol in the presence of air oxygen. The reaction mixture contained 0.05 mL of enzyme solution and 2.95 mL of 10 mM substrate solution prepared in 50 mM Tris-HCl, pH 7.0 and at 25°C. The blank sample contained only 3.0 mL of substrate solution. Enzyme activity was calculated from the linear portion of the curve. One unit of catecholase activity was defined as the amount of enzyme leading to an increase in absorbance of 0.001 per min [Citation18].
Cresolase activity of artichoke polphenol oxidase was determined by following the production of L-DOPA utilizing a modification of procedure reported by Arnow [Citation19]. Unless otherwise stated, the reaction mixture contained 5 mL of 2.5 mM L-tyrosine, 5 mL of 2.5 mM L-ascorbate dissolved 0.1 N Tris-HCl buffer, pH 7.0, an appropriate amount of the enzyme (soluble enzyme 50 μL; alginate beads 0.5 gr). This mixture was kept in a shaking water bath and the reaction was carried at 22 °C for 30 min. For estimating the amount of l-DOPA produced to a suitable aliquot (generally 1 mL) of the reaction mixture, 1 mL solutions of each of the following reagents were added sequentially: 2 M hydrochloric acid, 2 M sodium hydroxide, 15% (w/v) sodium molybdate, and finally 15% (w/v) of sodium nitrite. The absorbance at 460 nm was recorded after 1 hour. Standard curve of l-DOPA was drawn in the range of 0.1–3.0 mol. Activities of enzymes were determined using the slope of the calibration curve [Citation12].
Protein concentration was determined by the method of Lowry with bovine serum albumin as standard [Citation20].
All activity and protein assays were expressed as the mean of at least three different samples.
Enzyme Immobilization
The procedure of Palmieri et al. was employed. 5 mL of the enzyme preparation, 100 mg of alginate was added so as to obtain a 2% solution of sodium alginate. The mixture obtained was extruded drop-wise through a syringe (0.8 mm diameter) into a gently stirred 2 % (w/v) CuCl2 solution at 4 °C. After 2 h the formed beads containing enzyme were separated from the CuCl2 solution by filtration. They were washed on a filter two times with cold distilled water and stored in 0.1 N Tris-HCl buffer (pH 7.0) for further use [Citation7].
The filtered CuCl2 solution and the two washings were collected for protein determination.
Immobilization efficiency was defined as follows:
Where aimm is specific activity of immobilized enzyme (U/mg protein) and afree specific activity of free enzyme (U/mg protein) [Citation7].
Protein contents of free enzyme and washings were determined using the method of Lowry [Citation20].
Optimization of Immobilization Parameters
Various amounts of sodium alginate (1–4 %, w/v) were added to the gel mixture to study the effect of sodium alginate concentration on bead permeability. The formation of beads was carried out by a 2 % (w/v) CuCl2 solution.
In order to harden the solution, 2% (w/v) CuCl2 and CaCl2 solutions were assayed. After that, to also investigate the effect of CuCl2 concentration on the hardness of beads the 3% (w/v) sodium alginate solution was extruded drop-wise into different CuCl2 concentrations from 1 to 4 % (w/v).
The effect of bead size on the activity was investigated by altering the diameters of the syringe needle used (0.45, 0.8, 2, and 3 mm diameter).
Different enzyme loading in the beads was examined by incorporating various amounts of artichoke polyphenol oxidase (4.25, 4.75, and 6.5 units for cresolase activity and (3610, 4240, and 5815 units for catecholase activity) in 1 mL aqueous sodium alginate 3 % (w/v) solution.
The effect of bead amounts on the activity was determined by using various amounts (0.1, 0.2, 0.25, and 0.5 g beads).
Repeated Use of Polyphenol Oxidase Immobilized in the Alginate Beads
In order to test the reuse of entrapped polyphenol oxidase, the activities in the beads were assayed several times. After each polyphenol oxidase activity assay of the beads, they were removed, washed thoroughly with distilled water, and stored at 4°C. Then the beads were reassayed for both cresolase and catecholase activities, and the same process was repeated until the tenth use.
RESULTS AND DISCUSSION
Polyphenol oxidase isolated from artichoke head was immobilized within copper-alginate beads. It has been reported that an artichoke head has high polyphenol oxidase activity [Citation21–23]. It has also been thought that immobilized polyphenol oxidase could be used in the production of l-DOPA. l-DOPA is the drug of choice for treatment of Parkinson's disease, and currently it is produced chemically. Recent research has centered upon microbiological production of l-DOPA from Escherichia coli and Erwinia herbicola and upon the feasibility of l-DOPA production from tyrosinase (PPO) [Citation5]. In this study, cresolase activity of immobilized artichoke PPO was investigated for this purpose.
Entrapment, one of the immobilization techniques, can be defined as physical restriction of the enzyme within a confined space or network. Gelation of polyanionic or polycationic polymers by the addition of multi-valent counter-ions is a simple and common method of enzyme entrapment. Alginates are one of the most frequently used polymers due to their mild gelling properties and non-toxicity. Alginate is an anionic linear copolymer composed of 1,4′-linked β-D-mannuronic acid and α-L-guluronic acid in different proportions and sequential arrangements [Citation14]. In the present study, entrapment of artichoke polyphenol oxidase was carried out by drop-wise addition of sodium alginate (3 %, w/v) to CuCl2 (2 %, w/v) solution. It was determined that immobilization efficiency of polyphenol oxidase was approximately 70 %.
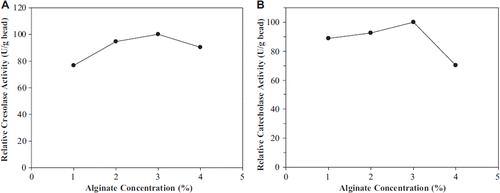
Alginate and CaCl2 concentration are major parameters for general enzyme entrapment because the cross-linking between alginate and divalent cations such as Ca2+, Cu2+, and Ba2+ leads to gelation and production of more stable gels. Therefore, the effects of alginate and divalent cation concentration on polyphenol oxidase activity were first investigated. Since Cu2+ has been widely used for immobilization of polyphenol oxidase, CuCl2 was used as the cross-linking agent as well as CaCl2 in this study. When alginate concentration was increased from 1 to 4 % (w/v), the highest polyphenol oxidase activity was found to be 3 % (w/v) for both cresolase activity and catecholase activity ().
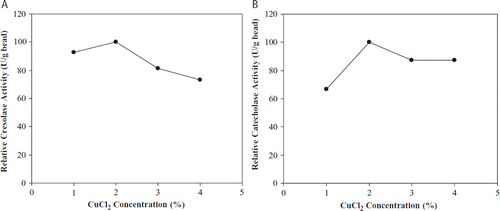
CuCl2 and CaCl2 were assayed as the cross-linking agent in the study. When we used CuCl2 solution as the hardening solution, higher enzymatic activities were observed (). It was known that alginate has the higher affinity for Cu2+ with respect to Ca2+. It may be explained that Cu2+ ion is a cofactor of polyphenol oxidase. Four concentrations of CuCl2 were tested, and the best polyphenol oxidase activity was observed at 2 % (w/v) ().
Table 1. The determination of hardening solution in immobilization of artichoke PPO within alginate beads
It was reported that alginate and CuCl2 concentration in the polyphenol oxidase entrapment changed in the range of 1–3 % (w/v) and 2–4 % (w/v), respectively [Citation3,Citation4,Citation7,Citation12]. Won et al. reported that an increase in alginate and hardening solution concentration will make it difficult for the enzyme to leak out of the gel network because alginate anions are cross-linked to divalent cations to form gel beads [Citation24].
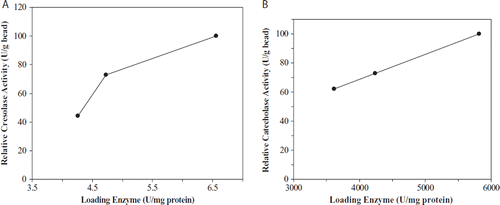
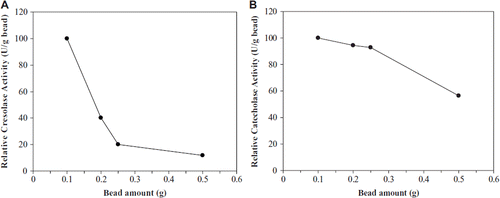
Initial enzyme loading of beads affects the efficiency of the immobilized system. In order to determine the effect of polyphenol oxidase loading in alginate beads on the activity, various concentrations of immobilized polyphenol oxidase (4.25, 4.75, and 6.5 units/mL of reaction volume) for cresolase activity were investigated while 3610, 4240, and 5815 U/mL of reaction volume were investigated for catecholase activity. As seen in , the highest activity was found at the loading enzyme concentration of 6.5 U/mL and 5815 U/mL for cresolase and catecholase activities, respectively. It was seen that both polyphenol oxidase activities increased depending on the increased enzyme concentration.
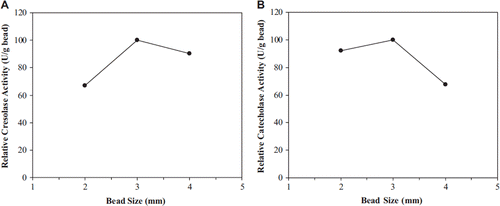
Another factor affecting rate of enzymatic reaction was bead size. Bead size determines the suitability of reactor configuration [Citation25]. It is expected that smaller beads will show higher catalytic activity due to reduced substrate transfer resistance [Citation24]. In this study, the alginate beads of four different sizes were generated by changing the diameter of the syringe needle. The average bead sizes measured by a calliper were 2, 3 and 4 mm. Highest polyphenol oxidase activity was attained when beads of mean diameter were 3 mm ().
When the effect of bead amount on the enzymatic activity was investigated, maximum activity was realized with 0.1 g of beads (). The lower enzyme activities were obtained at high bead amounts.
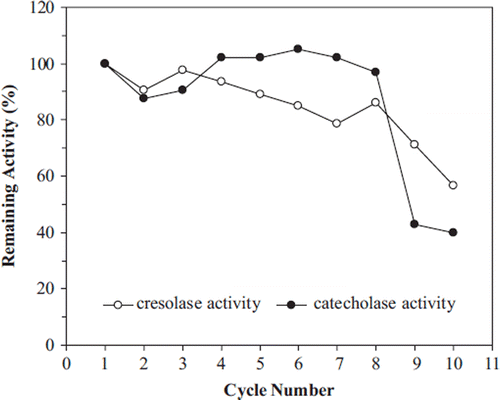
The reusing of immobilized enzymes is also one of the most important factors affecting the utilization of the immobilized enzymes. The reuse of the immobilized polyphenol oxidase from artichoke was studied in repeated batch processes. Both activities of immobilized artichoke polyphenol oxidase in copper alginate beads remained almost unchanged during the first eight cycles. 40 % of initial catecholase activity was retained up to ten cycles while 56 % of initial cresolase activity was retained (). After eight cycles, decreasing in the enzyme activity may be due to enzyme denaturation and due to physical loss of enzyme from the carrier.
CONCLUSION
Polyphenol oxidase was isolated from an artichoke head, and was successfully immobilized in copper alginate beads. Effects of immobilization conditions on the cresolase and catecholase activities of artichoke polyphenol oxidase were investigated. The optimum immobilization conditions were determined to be alginate and CuCl2 concentrations of 3 % (w/v) and 2%. Optimum bead size was 3 mm, and optimum bead amount was 0.25 g for maximum polyphenol oxidase activities. The polyphenol oxidase used in this study exhibited successful reuse for both activities. It maintained its initial activity after 8-time uses with both cresolase and catecholase activities. Supplementary experiments may be needed to improve the use of this immobilized preparation for biotechnological aims.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Mammeralla, E.J., Rubiolo, A.M. (2005). Study of the deactivation of β-galactosidase entrapped in alginate-carrageenan gels. Journal of Molecular Catalysis: Enzymatic 34: 7–13.
- Sahin, F., Demiral, G., Tümtürk, H. (2005). A novel matrix for the immobilization of acetylcholinesterase. Int. J. Biol. Macromol. 37: 148–153.
- Ates, S., Cortenlioglu, E., Bayraktar, E., Mehmetoglu, U. (2006). Production of L-DOPA using Cu-alginate gel immobilized tyrosinase in a batch and packed bed reactor. Enzyme Microb. Technol 40(4): 683–687.
- Palmieri, G., Giardina, P., Desiderio, B., Marzullo, L., Giamberini, M., Sonnia, G. (1994). A new enzyme immobilization procedure using cupper alginate gel: Application to a fungal phenol oxidase. Enzyme Microb. Technol. 16: 151–158.
- Pialis, P., Jimenez Hamann, M.C., Saville, B.A. (1996). L-DOPA production from tyrosinase immobilized on Nylon 6,6. Biotech Bioeng. 51: 141–147.
- Carvalho, G.M.J., Alves, T.L.M., Freire, D.M.G. (2000). L-DOPA production by immobilized tyrosinase. Appl Biochem Biotechnol. 84–86: 791–800.
- Munjal, N., Sawhney, S.K. (2002). Stability and properties of mushroom tyrosinase entrapped in alginate, polyacrylamid, and gelatin gels. Enzyme Microb. Technol. 30: 613–619.
- Seetharam, G., Saville, B.A. (2002). L-DOPA production from tyrosinase immobilized on zeolite. Enzyme Microb. Technol. 1: 747–753.
- Pialis, P., Saville, B.A. (1998). Production of L-DOPA from tyrosinase immobilized on Nylon 6,6: Enzyme stability and scaleup. Enzyme Microb. Technol. 22: 261–268.
- Ho, P.Y., Chiou, M.S., Chao, A.C. (2003). Production of L-DOPA by tyrosinase immobilized on modified polystyrene. Appl Biochem Biotechnol. 111: 139–152.
- Vilanova, E., Manjon, A., Iborra, J. (1984). Tyrosine hydroxylase activity of immobilized tyrosinase on Enzacryl-AA and CP-AA supports: Stabilization and properties. Biotechl Bioeng. 26: 1306–1312.
- Yahsi, A., Sahin, F., Demirel, G., Tümtürk, H. (2005). Binary immobilization of tyrosinase by using alginate gel beads and poly(acrylamide-co-acrylic acid) hydrogels, International Journal of Biological Molecules 36: 253–258.
- Yıldız, H.B., Toppare, L., Hepuzer Gursel, Y., Yagci, Y. (2006). Immobilization of polyphenol oxidase in conducting graft copolymers and determination of phenolic amount in red wines with enzyme electrodes. Enzyme Microb. Technol. 39(4): 945–948.
- Sarkar, J.M., Leonowicz, A., Bollog, J.M. (1989). Immobilization of enzymes on clays and soils. Soil Biol. Biochem. 21(2): 223–230.
- Batra, R., Gupta, M.N. (1994). Non-covalent immobilization of potato (Solanum tuberosum) polyphenol oxidase on chitin. Biotech. Appl. Biochem. 19: 209–215.
- Estrada, P., Sanchez-Muniz, R., Acebal, C., Arche, R., Castillon, M.P. (1991). Characterization and optimization of immobilized polyphenol oxidase in low water organic solvents. Biotech. Appl Biochem. 14: 12–20.
- Yagar, H., Sagiroglu, A. (2002). Partially purification and characterization of polyphenol oxidase of quince. Turk J Chem. 26(1): 97–103.
- Oktay, M., Küfrevioğlu, I., Kocaçalişkan, I., Şakiroğlu, H. (1995). Polyphenol oxidase from Amasya apple. J Food Science 60: 18–20.
- Arnow, L.E. (1937). Colorimetric determination of components of 3-dihydroxyphenylalanine-tyrosine mixtures. J. Biol. Chem. 118: 531–537.
- Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J. (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275.
- Espin, J.C., Tudela, J., Canovas, F.G. (1997). Monophenolase activity of polyphenol oxidase from artichoke (Cynara Scolymus L.) heads. Lebensm.-Wiss. U.-Technol. 30: 819–825.
- López-Molina, D., Hiner, A.N.P., Tudela, J., García-Cánovas, F., Rodríguez-López J.N. (2003). Enzymatic removal of phenols from aqueous solution by artichoke (Cynara Scolymus L.) extracts. Enzyme Microb. Technol. 33(5): 738–742.
- Aydemir, T. (2004). Partial purification and characterization of polyphenol oxidase from artichoke (Cynara Scolymus L.) heads. Food Chem. 87(1): 59–67.
- Won, K., Kim, S., Kim, K.J., Park, H.W., Moon, S.J. (2005). Optimization of lipase entrapment in Ca-alginate gel beads. Proc. Biochem. 40: 2149–2154.
- Dey, G., Singh, B., Banerjce, R. (2003). Immobilization of α-amylase produced by Bacillus circulans GRS 313. Braz. Arch. Biol. Techn. 46(2): 167–176.