Abstract
Abstract: The effect of transfusion of PEGylated hemoglobin (PEG-Hb) was evaluated in anesthetized rats subjected to 2 hours of focal cerebral ischemia and 1 day of reperfusion. PEG-Hb was stored in the carboxy state (PEG-COHb) to reduce autooxidation and increase the shelf life. Transfusion of 10 ml/kg of PEG-COHb at 20 minutes of ischemia did not alter arterial blood pressure or increase red cell flux in the ischemic core. Plasma hemoglobin increased to only 0.6 g/dL, yet infarct volume was markedly decreased and neurological deficits were improved. We conclude that early topload transfusion of PEG-COHb protects the brain from ischemic stroke.
INTRODUCTION
Occlusion of a major cerebral artery produces an ischemic core that undergoes rapid neurodegeneration and a penumbral region with less intense ischemia and with delayed neurodegeneration. Anastomotic vessels between the territories of the major cerebral arteries permit residual blood flow that sustains neuronal viability for several hours in the penumbra. Thus, the acute stage of ischemic stroke is amenable to oxygen therapeutics. Transfusion of cell-free hemoglobin (Hb) into the plasma affords the opportunity to promote oxygenation in the ischemic region and possibly reduce damage from stroke. Indeed, hypervolemic exchange transfusion of ααcrosslinked tetrameric Hb soon after the onset of experimental stroke was reported to reduce infarct volume in rats [Citation1], but a clinical trial with this compound was not effective, possibly because the time to initial treatment was as long as 18 hours [Citation2]. In addition, αα-crosslinked tetrameric Hb readily extravasates in the peripheral circulation where it can scavenge nitric oxide (NO) and cause hypertension [Citation3]. Hypervolemic exchange transfusion of larger polymers of Hb that extravasate much less and do not impair endothelial-dependent dilation or cause hypertension [Citation4] have also been found to reduce infarct volume from experimental stroke [Citation5–7]. Likewise, transfusion of Hb encapsulated in vesicles was reported to be effective in experimental stroke [Citation8].
As with polymeric Hb, polyethylene glycol-conjugated Hb (PEG-Hb) has an increased molecular radius and does not readily extravasate or cause hypertension [Citation3,Citation9–11]. Furthermore, conjugation of the Hb molecule surface with PEG reduces immunogenicity, increases oncotic pressure, and increases plasma viscosity [Citation12–14]. Maintaining plasma viscosity is thought to be important for maintaining shear stress-induced production of NO by the endothelium and thereby promote vasodilation [Citation15]. Therefore, PEG-Hb may provide several advantages over polymeric Hb.
Little work has been done with PEGylated Hb in experimental stroke [Citation16]. Moreover, studies of crosslinked and polymerized Hb in stroke models used an exchange transfusion to augment the plasma Hb concentration. However, a topload transfusion would be simpler to implement clinically. In the present study, we evaluated the effect of a topload transfusion of PEG-Hb during middle cerebral artery occlusion (MCAO) on infarct volume in the rat. PEG-Hb was stored in the carboxy state (PEG-COHb) to reduce autooxidation and increase the shelf life. Comparisons were made to control groups that were transfused with PEG-bovine serum albumin or that received no transfusion.
METHODS
Surgical Preparation
All procedures were approved by the Johns Hopkins University Animal Care and Use Committee. Anesthesia was induced in male Wistar rats (250–350 g) with 5% isoflurane and maintained with approximately 2% isoflurane via nose cone with spontaneous ventilation during surgery. Isoflurane concentration was decreased to approximately 1.5% after surgery. Inspired O2 was approximately 25–30%. Catheters were inserted into a femoral vein for transfusion and into a femoral artery for measuring arterial blood pressure and sampling arterial blood. A heating lamp was used to maintain rectal temperature during ischemia and early reperfusion. A small incision was made in the scalp and a small burr hole was made in the lateral parietal bone until only a thin amount of bone remained. A 1-mm diameter fiberoptic probe was secured against the thinned bone. The probe was connected to a laser-Doppler flowmeter, which transmits and receives near-infrared light and calculates relative changes in red blood cell flux. The laser-Doppler flux signal was used to assess the adequacy of vascular occlusion during the ischemic period.
Transient focal cerebral ischemia was induced by the intraluminal filament technique to occlude blood flow into the middle cerebral artery [Citation17]. The right common carotid artery was exposed through a lateral incision and occluded. The right external carotid artery was dissected and ligated and the occipital artery branch of the external carotid artery was isolated and coagulated. The proximal pterygopalatine artery branch of the right internal carotid artery was ligated, and a 4-0 monofilament nylon suture (with the tip rounded) was advanced approximately 2 cm into the internal carotid artery. The filament position was adjusted to produce at least a 60% reduction in the laser-Doppler flux signal. After two hours of occlusion, reperfusion was begun by withdrawing the intraluminal suture. After monitoring for the first 30 min of reperfusion, catheters were removed, incisions were closed with suture, and anesthesia was discontinued.
Experimental Design
PEG-albumin and PEG-COHb were synthesized at Prolong Pharmaceuticals (South Plainfield, New Jersey). Surface lysine residues on purified bovine Hb were conjugated with 5000 molecular weight PEG [Citation18]. The PEG-Hb solution was bubbled with CO to convert >80% of the PEG-Hb to PEG-COHb before storage. Solutions containing 4–6% protein were stored at 2–10°C in sterile blood bags. Three groups of 10 rats were studied: 1) no transfusion; 2) bovine PEG-albumin transfusion; and 3) bovine PEG-COHb transfusion. On the day of the experiment, an aliquot of the solution was warmed and transfused as a topload equivalent to 10 ml/kg body weight. The transfusion started at 20 min of MCAO. The rate of intravenous transfusion was 0.5 ml/min and occurred over approximately 5–7 min.
Mean arterial pressure and the percent change in laser-Doppler flow was recorded at 15-min intervals during 2 h of MCAO and 30 min of reperfusion. Rectal temperature was monitored through 1 h of reperfusion as the animals recovered from anesthesia in a warm environment. Arterial blood (∼0.7 ml) was sampled at baseline, 1 h of MCAO, and 30 min of reperfusion. Arterial blood pH, PCO2, PO2, and electrolytes were measured on a Radiometer blood gas analyzer (ABL80). Arterial Hb concentration, O2 saturation, MetHb, and COHb were measured on a Radiometer Hemoximeter (OSM3). Plasma from the samples was analyzed for Hb concentration. The rats were assessed for neurologic deficits at 1 and 24 h of reperfusion on a 0–4 scale (0 = no deficit, 1 = failure to extend forelimb during placing, 2 = circling, 3 = unilateral weakness, and 4 = no spontaneous motor activity). At 24 h, the brain was harvested to measure infarct volume. The brain was divided into 7 coronal sections (2 mm thick). The sections were stained with a 1% solution of triphenyltetrazolium chloride, which stains viable regions red. The pale, nonviable areas of cerebral cortex and striatum were measured on the anterior and posterior surfaces of each section. The infarct volume of each section was calculated from the product of the section thickness and the average of the infarct area on the anterior and posterior surfaces. Total infarct volume for cortex and striatum was obtained by summing the volume from each section. Values are expressed as a percent of the entire structure.
Measurements were compared among the 3 groups by ANOVA and the Newman-Keuls multiple range test at the 0.05 level of significance. Data are presented as mean ± SE.
RESULTS
Arterial pH, PCO2, and PO2 remained stable and in the physiologic range during MCAO and early reperfusion in all groups of rats (). There were no significant differences among the groups transfused at 20 min of MCAO. Electrolyte concentrations remained similar among the groups after transfusion (). As expected with a topload protein transfusion, small decreases in hematocrit occurred (). However, there were no significant differences in hematocrit or whole blood Hb concentration in the PEG-COHb-transfused group compared to the PEG-albumin-transfused group. The percents of MetHb and COHb in whole blood were slightly elevated after transfusion of PEG-COHb (). Analysis of plasma Hb at 60 min of MCAO (about 35 min after completing the PEG-COHb transfusion) indicated concentrations of 0.6 ± 0.1 g/dL (±SE), and the level remained relatively unchanged at 30 min of reperfusion. The percent of COHb in the plasma Hb was in the 11 ± 4% at 60 min of MCAO and 6 ± 1% at 30 min of reperfusion.
Table 1. Arterial pH and blood gases during and after 2 h of middle cerebral artery occlusion (MCAO) in groups with no transfusion or transfusion at 20 min of MCAO (mean ± SE; n = 10)
Table 2. Arterial blood electrolytes during and after 2 h of MCAO in groups with no transfusion or transfusion at 20 min of MCAO (mean ± SE; n = 10)
Table 3. Whole blood hemoglobin analysis during and after 2 h of MCAO in groups with no transfusion or transfusion at 20 min of MCAO (mean ± SE; n = 10)
Values for mean arterial blood pressure are shown in . Although there was tendency for pressure to increase slightly during prolonged MCAO in the PEG-COHb group, the values were not significantly different among groups at any time point. Laser-Doppler flow was monitored over the lateral cortex where ischemia is most dense. Values are displayed in . In all groups, flow decreased to approximately 25% of baseline initially and increased slightly over time to approximately 30% of baseline. With reperfusion, flow was restored to approximately 80% of baseline in all groups. There were no significant differences among groups at any time point. Rectal temperature was maintained in the range of 36.5–37.0 °C during ischemia and at 1 h of reperfusion after the rats awoke from anesthesia (). Moreover, the rats did not display fever at 24 h.
Figure 1. Mean arterial blood pressure (A) and laser-Doppler flux, measured over lateral parietal cortex and expressed as a percent of baseline (B), during 2 h of middle cerebral artery occlusion (MCAO) and the first 30 min of reperfusion in groups of rats undergoing either no transfusion or transfusion with 10 ml/kg of PEG-albumin or PEG-COHb at 20 min of MCAO (mean ± SE; n = 10 per group).
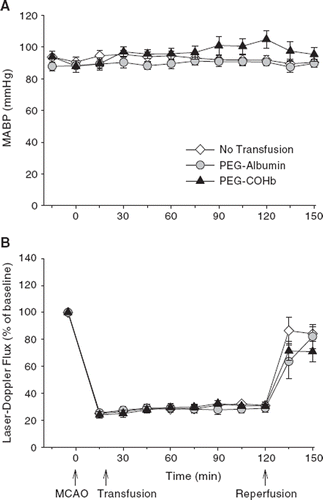
Table 4. Rectal temperature during and after 2 h of MCAO in groups with no transfusion or transfusion at 20 min of MCAO (mean ± SE; n = 10)
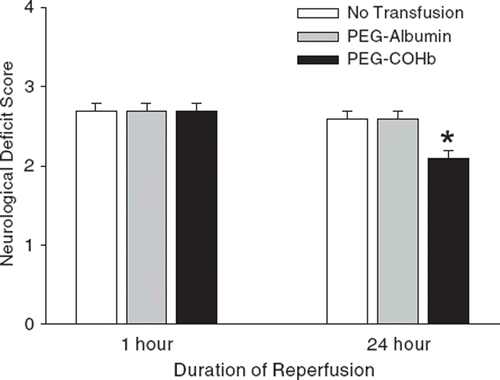
After awakening from anesthesia, neurologic deficit scores were similar among groups (). However, upon retesting at 24 h, neurologic deficit scores improved selectively in the PEG-COHb group.
Figure 2. Neurologic deficit score on a 0–4 scale (0 = no deficit) at 1 or 24 h of reperfusion after 2 h of MCAO in groups with no transfusion or transfusion of PEG-albumin or PEG-COHb at 20 min of MCAO (mean ± SE; n = 10). *P < 0.05 between PEG-COHb groups versus no transfusion and PEG-albumin groups.
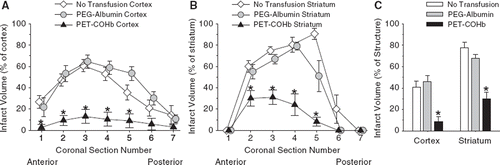
Values for infarct volume at each coronal level and total infarct volumes are shown in . Infarct volume was similar in the group without transfusion and the group transfused with PEG-albumin. Infarct volume was significantly reduced in the group transfused with PEG-COHb compared to either the non-transfused or PEG-albumin group. In the PEG-COHb group, infarct volume was reduced by 82% in cortex and 56% in striatum relative to the PEG-albumin group. The reduction in infarct volume was present in each of the 5 most anterior slices, thereby indicating that tissue rescue was widespread over the entire middle cerebral artery distribution area.
Figure 3. Infarct volume in each of the 7 coronal sections for cerebral cortex (A) and striatum (B), and total infarct volume summed over the 7 sections for cerebral cortex and striatum (C). Values are expressed as a percent of the contralateral total structure (means ± SE; n = 10). *P < 0.05 between PEG-COHb group versus no transfusion and PEG-albumin groups.
DISCUSSION
The degree of tissue rescue by PEG-COHb topload transfusion during transient focal cerebral ischemia is substantially greater than that reported with hypervolemic exchange transfusion with αα-crosslinked Hb in rats [Citation1] or polymeric Hb in mice [Citation6,Citation7]. This finding is remarkable because the plasma Hb concentration attained with the 10 ml/kg topload was 0.6 g/dL, which is considerably less than the 2–2.5 g/dL typically attained with 30–40% exchange transfusion of fluids containing 6 g/dL of Hb [Citation19,Citation20]. Moreover, increasing the plasma concentration of αα-crosslinked Hb further by increasing the concentration of Hb in the transfusion fluid from 10 g/dL to 20 g/dL produces additional reductions in infarct volume [Citation21]. These comparisons suggest that PEG-COHb may be superior per mole of heme compared to αα-crosslinked Hb and polymeric Hb in rescuing the brain from stroke, although we cannot exclude differences arising from the exchange transfusion protocol or differences between mouse and rat. Nevertheless, the results indicate that large plasma concentrations of PEGylated COHb are not required to rescue the brain. The theoretical advantage of exchange transfusion is that whole blood viscosity can be reduced by decreasing hematocrit and thereby promote collateral blood flow. The advantage of the topload over the exchange transfusion is that this protocol would be easier to implement in clinical stroke and the 10 ml/kg volume is readily tolerated without producing marked hypervolemic-induced hypertension. Significant hypertension was not observed in the present study.
Rescue by the PEG-COHb did not appear to be related to an increase in blood flow in the ischemic core of cortex as assessed by laser-Doppler flowmetry at a single site. However, it is possible that collateral blood flow was improved in the ischemic border region sufficient to salvage tissue. The viscosity of the PEGylated Hb solution is closer to that of whole blood compared to solutions of crosslinked and polymeric Hb and thus may better maintain endothelial shear stress and associated NO production, which could help maintain dilation of collateral arteries.
The 10-ml/kg topload transfusion of PEG-albumin and PEG-COHb produced approximately 8–10% decreases in hematocrit. This relatively small decrease in hematocrit will have only a small effect on blood viscosity and is unlikely to improve perfusion sufficiently to reduce brain injury by reducing blood viscosity. The lack of a difference in infarct volume between the group transfused with PEG-albumin and the group with no transfusion in this study and in a previous study with greater hemodilution [Citation7] supports this premise. Moreover, the increase in plasma [Hb] to 0.6 g/dL after PEG-COHb topload was inadequate to offset the decrease in hematocrit. Thus, whole blood [Hb] and arterial O2 content were not increased by PEG-COHb transfusion. However, even in the absence of an increase in arterial O2 content or blood flow, an O2 carrier in the plasma may be capable of enhancing O2 delivery to ischemic tissue for several reasons. First, oxygen solubility in plasma is low and represents a major site of resistance of O2 diffusion between the red cell and mitochondria in the tissue. By carrying a large amount of O2 in the plasma, O2 transport from the red cell membrane to the endothelium is facilitated. In this regard, efficacy of transfusion of Hb polymers during MCAO is lost when the solution primarily contains polymers of molecular weight greater than 14 MDa [Citation7]. The current bovine PEG-Hb with approximately 8–10 side chains of 5000 molecular weight PEG may represent a good balance of being small enough to have high mobility for facilitating O2 transport in the plasma but large enough to limit extravasation. Second, red cell flux through individual microvessels is heterogeneous and this heterogeneity is amplified under conditions of low perfusion pressure during incomplete ischemia. By delivering O2 through the flow of plasma in capillaries that are red cell deprived, O2 delivery may become more homogenous among capillaries. Third, red cells are particulate and their surface area does not cover the entire surface area of capillary endothelium at any one instant. An O2 carrier in the plasma increases the effective surface area for O2 diffusion. Therefore, a topload infusion of cell-free Hb could invoke several mechanisms of O2 delivery independent of bulk arterial O2 content and macroscopic blood flow.
Another consideration is that the PEG-Hb product was transfused in the carboxy state. Vandergriff et al. [Citation22] reported that mixing human PEG-COHb with red blood cells resulted in equilibration of the percent of COHb in each compartment at 0.5 h after mixing. In the present study using ∼80% COHb in the stock solution of PEG-Hb, the COHb in the plasma Hb was in the 10% range at 0.5 h after transfusion and whole blood COHb was ∼2%. Thus, red blood cell Hb and the rest of the body provided a large sink for CO. The residual amount of 2% COHb in whole blood has only a minor effect on oxygen carry capacity. Thus, most of the transfused PEG-COHb can act as an O2 carrier in a relatively short timeframe after transfusion.
In addition, part of the brain protection from ischemia seen with PEG-COHb might be attributed to release of CO. Small molecules that release CO have been shown to exert vasodilatory, anti-inflammatory, and anti-apoptotic properties in a variety of models in the peripheral circulation [Citation23] and to protect the neonatal brain vasculature from seizure-induced dysfunction [Citation24]. Moreover, transfusion of red blood cells containing high COHb was shown to be beneficial in limiting microvascular markers of apoptosis after hemorrhagic shock [Citation25]. Furthermore, combined pretreatment and post-treatment with human PEG-COHb has been shown to protect the heart from coronary artery occlusion in rats [Citation22]. Thus, the use of PEG-COHb to release a well-defined amount of CO may have potential benefit in ischemic tissue. Future work is required to define the role of CO and its mechanism of action in the protection afforded by PEG-COHb. Interestingly, small amounts of PEG-Hb can protect the brain from ischemia when the PEG-Hb is transfused in the S-nitrosylated form [Citation16,Citation26]. Thus, transfusion of PEG-Hb in either the carboxy or S-nitrosylated form may enhance the efficacy in cerebral ischemia.
In summary, the present work provides a proof of principle that PEG-COHb has potential as a therapy in ischemic stroke. Further work is required to determine the therapeutic time window of opportunity for administration, the optimal transfusion protocol and dose, long-term effects on neurobehavior, efficacy in a model of more prolonged ischemia, and a possible role of CO released from the Hb.
Declaration of interest: J.A. Klaus, K.K. Kibler, and R.C. Koehler have no financial interest in Prolong Pharmaceuticals and report no conflict of interest. Abraham Abuchowski is CEO of Prolong Pharmaceuticals, which holds the IP for bovine PEG-Hb.
References
- Cole, D.J., Drummond, J.C., Patel, P.M., and Reynolds, L.R. (1997). Hypervolemic-hemodilution during cerebral ischemia in rats: effect of diaspirin cross-linked hemoglobin (DCLHb) on neurologic outcome and infarct volume. J Neurosurg Anesthesiol 9:44–50.
- Saxena, R., Wijnhoud, A.D., Carton, H., Hacke, W., Kaste, M., Przybelski, R.J., Stern, K.N., and Koudstaal, P.J. (1999). Controlled safety study of a hemoglobin-based oxygen carrier, DCLHb, in acute ischemic stroke. Stroke 30:993–996.
- Tsai, A.G., Cabrales, P., Manjula, B.N., Acharya, S.A., Winslow, R.M., and Intaglietta, M. (2006). Dissociation of local nitric oxide concentration and vasoconstriction in the presence of cell-free hemoglobin oxygen carriers. Blood 108:3603–3610.
- Matheson, B., Kwansa, H.E., Bucci, E., Rebel, A., and Koehler, R.C. (2002). Vascular response to infusions of a nonextravasating hemoglobin polymer. J Appl Physiol 93:1479–1486.
- Bobofchak, K.M., Mito, T., Texel, S.J., Bellelli, A., Nemoto, M., Traystman, R.J., Koehler, R.C., Brinigar, W.S., and Fronticelli, C. (2003). A recombinant polymeric hemoglobin with conformational, functional, and physiological characteristics of an in vivo O2 transporter. Am J Physiol Heart Circ Physiol 285:H549–H561.
- Nemoto, M., Mito, T., Brinigar, W.S., Fronticelli, C., and Koehler, R.C. (2006). Salvage of focal cerebral ischemic damage by transfusion of high O2-affinity recombinant hemoglobin polymers in mouse. J Appl Physiol 100:1688–1691.
- Mito, T., Nemoto, M., Kwansa, H., Sampei, K., Habeeb, M., Murphy, S.J., Bucci, E., and Koehler, R.C. (2009). Decreased damage from transient focal cerebral ischemia by transfusion of zero-link hemoglobin polymers in mouse. Stroke 40:278–284.
- Kawaguchi, A.T., Fukumoto, D., Haida, M., Ogata, Y., Yamano, M., and Tsukada, H. (2007). Liposome-encapsulated hemoglobin reduces the size of cerebral infarction in the rat: evaluation with photochemically induced thrombosis of the middle cerebral artery. Stroke 38:1626–1632.
- Conover, C.D., Malatesta, P., Lejeune, L., Chang, C.L., and Shorr, R.G. (1996). The effects of hemodilution with polyethylene glycol bovine hemoglobin (PEG-Hb) in a conscious porcine model. J Investig Med 44:238–246.
- Conover, C.D., Lejeune, L., Shum, K., Gilbert, C., and Shorr, R.G. (1997). Physiological effect of polyethylene glycol conjugation on stroma-free bovine hemoglobin in the conscious dog after partial exchange transfusion. Artif Organs 21:369–378.
- Young, M.A., Malavalli, A., Winslow, N., Vandegriff, K.D., and Winslow, R.M. (2007). Toxicity and hemodynamic effects after single dose administration of MalPEG-hemoglobin (MP4) in rhesus monkeys. Transl Res 149:333–342.
- Nucci, M.L., Shorr, R.G.L., and Abuchowski, A. (1996). PEG-Hemoglobin-Oxygen carrying blood substitute. Drugs of the Future 21:29–32.
- Intaglietta, M. (1999). Microcirculatory basis for the design of artificial blood. Microcirculation 6:247–258.
- Wettstein, R., Tsai, A.G., Erni, D., Winslow, R.M., and Intaglietta, M. (2003). Resuscitation with polyethylene glycol-modified human hemoglobin improves microcirculatory blood flow and tissue oxygenation after hemorrhagic shock in awake hamsters. Crit Care Med 31:1824–1830.
- Tsai, A.G., Acero, C., Nance, P.R., Cabrales, P., Frangos, J.A., Buerk, D.G., and Intaglietta, M. (2005). Elevated plasma viscosity in extreme hemodilution increases perivascular nitric oxide concentration and microvascular perfusion. Am J Physiol Heart Circ Physiol 288:H1730–H1739.
- Kawaguchi, A.T., Nakai, K., Fukumoto, D., Yamano, M., Haida, M., and Tsukada, H. (2009). S-nitrosylated pegylated hemoglobin reduces the size of cerebral infarction in rats. Artif Organs 33:183–188.
- Alkayed, N.J., Harukuni, I., Kimes, A.S., London, E.D., Traystman, R.J., and Hurn, P.D. (1998). Gender-linked brain injury in experimental stroke. Stroke 29:159–166.
- Nho, K., Glower, D., Bredehoeft, S., Shankar, H., Shorr, R., and Abuchowski, A. (1992). PEG-bovine hemoglobin: safety in a canine dehydrated hypovolemic-hemorrhagic shock model. Biomater Artif Cells Immobilization Biotechnol 20:511–524.
- Rebel, A., Ulatowski, J.A., Joung, K., Bucci, E., Traystman, R.J., and Koehler, R.C. (2002). Regional cerebral blood flow in cats with cross-linked hemoglobin transfusion during focal cerebral ischemia. Am J Physiol Heart Circ Physiol 282:H832–H841.
- Ulatowski, J.A., Bucci, E., Nishikawa, T., Razynska, A., Williams, M.A., Takeshima, R., Traystman, R.J., and Koehler, R.C. (1996). Cerebral O2 transport with hematocrit reduced by cross-linked hemoglobin transfusion. Am J Physiol Heart Circ Physiol 270:H466–H475.
- Cole, D.J., Drummond, J.C., Patel, P.M., Nary, J.C., and Applegate, R.L. (1996). Effect of oncotic pressure of diaspirin cross-linked hemoglobin (DCLHb) on brain injury after temporary focal cerebral ischemia in rats. Anesth Analg 83:342–347.
- Vandegriff, K.D., Young, M.A., Lohman, J., Bellelli, A., Samaja, M., Malavalli, A., and Winslow, R.M. (2008). CO-MP4, a polyethylene glycol-conjugated haemoglobin derivative and carbon monoxide carrier that reduces myocardial infarct size in rats. Br J Pharmacol 154:1649–1661.
- Motterlini, R. (2007). Carbon monoxide-releasing molecules (CO-RMs): vasodilatory, anti-ischaemic and anti-inflammatory activities. Biochem Soc Trans 35:1142–1146.
- Zimmermann, A., Leffler, C.W., Tcheranova, D., Fedinec, A.L., and Parfenova, H. (2007). Cerebroprotective effects of the CO-releasing molecule CORM-A1 against seizure-induced neonatal vascular injury. Am J Physiol Heart Circ Physiol 293:H2501–H2507.
- Cabrales, P., Tsai, A.G., and Intaglietta, M. (2007). Hemorrhagic shock resuscitation with carbon monoxide saturated blood. Resuscitation 72:306–318.
- Otani, H., Jesmin, S., Togashi, H., Sakuma, I., Nakai, K., Satoh, H., Yoshioka, M., and Kitabatake, A. (2004). An S-nitrosylated hemoglobin derivative protects the rat hippocampus from ischemia-induced long-term potentiation impairment with a time window. J Pharmacol Sci 96:188–198.