Abstract
Abstract: In this study, resolution of (R,S)-1-phenyl 1-propanol by lipase-catalyzed enantioselective esterification was achieved. To investigate the effect of lipase type on enantiomeric excess, three different lipases were used. Novozym 435 exhibited the highest enantioselectivity for resolution of (R,S)-1-phenyl 1-propanol. The effects of carbon length of fatty acids from C12 to C16, which were used as acyl donor, organic solvents with Log P values from 0.5 to 4.5, acyl donor/alcohol molar ratio (1:1, 3:2, 2:1, 3:1), amount of added molecular sieves (0–133.2 kg/m3), and temperature (10–60°C) on the enantioselectivity were investigated. The best reaction conditions were comprised of using toluene (Log P= 2.5) as solvent, lauric acid (12C) as acyl donor, 133.2 kg/m3 molecular sieves at 50°C and acyl donor/alcohol molar ratio as 1:1. Under these conditions, the enantiomeric excess of S enantiomer ee (S) was obtained as 95% for a reaction time of 2.5 hours.
INTRODUCTION
Recently, the demand for enantiomerically pure compounds has rapidly increased in the pharmaceutical and fine chemical industries [Citation1,Citation2,Citation3]. This interest is mainly due to the awareness of different biological effects of the various forms of enantiomeric products. One enantiomer usually provides the desired activity while the opposite enantiomer may be inactive or sometimes toxic. Therefore, during the last decade there has been an increased trend towards the use of enzymes for asymmetric synthesis and kinetic resolution to obtain pure enantiomers. Lipases are frequently used both in kinetic resolutions of racemates and in asymmetrizations of prochiral and meso compounds [Citation4,Citation5,Citation6]. Lipase activity and selectivity are strongly influenced by the medium used for the desired reaction [Citation7]. Lipases successfully resolve chiral secondary alcohols.
Enantiomerically pure secondary alcohols are important synthetic intermediates and chiral auxiliaries [Citation8]. Lipases from Pseudomonas cepacia, Candida rugosa, and Candida antarctica have been used in the resolution of primary and secondary alcohols [Citation2,Citation3,Citation9,Citation10]. The acyl donor is used in the acyl transfer reaction either as an acid in esterification or as an ester in transesterification. The most commonly employed activated ester is vinyl acetate used in transesterification reactions [Citation8]. Acetaldehyde is the single by-product and is known to deactivate lipases [Citation3]. This has limited the use of vinyl ester as an acylating reagent. Some acyl donors generate nonharmful byproducts, but they are less reactive than vinyl acetate. Fatty acid is the conventional acyl donor in the esterification reaction. It is more stable chemically, less toxic, cheaper, and more readily available [Citation11].
The immobilized lipase-catalyzed resolution of (R,S)-1-phenyl ethanol in a recirculated packed bed reactor was investigated by Suan and Sarmidi [Citation3]. They used fatty acids as acyl donor and screened six commercial lipases. The enzyme activity and enantioselectivity were determined by varying substrate concentration from 25 to 50 mM, acyl length of fatty acid from C12 to C18, organic solvents with log P values from 1.4 to 4.5, and reaction temperature from 25 to 50°C. They found that the enzymes are highly selective toward the (R) enantiomer of the chiral alcohol. The initial reaction rates increased in the range from lauric acid to palmitic acid, but decreased about 3-4% for stearic acid. Holmquist et al. reported that the enantioselectivity of an enzyme is dependent on the carbon number of fatty acids [Citation12]. Ottoson and Hult reported that the enantioselectivity of C. antarctica lipase B was strongly influenced by the a chiral chain length of vinyl ester in the transesterification of 3-methyl-2-butanol [Citation13].
Direct enzymatic esterification catalyzed by immobilized Candida antarctica lipase (CALB) and Rhizomucor miehei lipase (RML) for the kinetic resolution of some primary alcohols with a chiral center at the next carbon atom was investigated [Citation11]. The reactions were performed in a solvent-free system with removal of water at low pressure. CALB was superior to RML in terms of both reaction rates and enantioselectivity. The highest enantioselectivity value was obtained for decanoic acid. The enantioselectivity of CALB increased with decreasing reaction temperature.
The choice of solvent is an important parameter for the application of lipase in racemate resolution. The solvent hydrophobicity, characterized by a log P value, had an effect on the activity of the catalyst. Log P above 2 has been suggested as the best value. Frings et al. reported that no dependency of enantioselectivity on log P could be detected in the kinetic resolution of 1-phenylethanol [Citation14]. Suan and Sarmidi found that the polarity of the solvent greatly affects the catalytic activity, but it seems not to influence the enzyme enantioselectivity [Citation3].
Temperature control is regarded as the simplest and most practical method for the lipase catalyzed kinetic resolution of the alcohols. Sakai demonstrated that the low temperature method was easily applied to the kinetic resolution of primary and secondary alcohols [Citation15]. For example, in the resolution of solketal, while a low enantiomeric ratio (E) (16) was obtained at 23°C, a high E (55) was obtained at −40°C. Temperature effect was investigated for the immobilized lipase-catalyzed resolution of (R,S)-1-phenylethanol in a recirculated packed bed reactor by Suan and Sarmidi [Citation3]. They observed that the enzymes were partially deactivated at high temperature and the enantioselectivities of the enzymes were not effected by the change in temperature.
There are a lot of studies about the kinetic resolution of primary and secondary alcohols by lipase catalyzed enantioselective transesterification reaction in the literature. However, there is no study about the kinetic resolution of racemic 1-phenyl-1-propanol by lipase catalyzed enantioselective esterification reaction. Tewari et al. studied the thermodynamics of the lipase-catalyzed transesterification of 1-phenyl-1-alkonols and butyl acetate in organic solvents [Citation16]. In this study, equilibrium measurments of the reaction with (R)-(+)-1-phenyl propanol and butyl acetate were carried out in n-hexane at 298.15 K. Bachu et al. investigated the influence of microwave irradiation on lipase-catalyzed kinetic resolution of racemic 1-phenyl-1-propanol and some racemic secondary alcohols [Citation17]. They used p-chlorophenylacetate as an acyl donor and toluene as the solvent for this tranesterification reaction. They obtained higher conversion values for microwave-assisted lipase kinetic resolution than for conventional heating.
In our study, the kinetic resolution of racemic 1-phenyl 1-propanol by lipase catalyzed enantioselective esterification reaction was investigated. Fatty acids were used as conventional acyl donors and water produced was removed using a molecular sieve. In order to define the optimum conditions for enantiomerically pure 1-phenyl 1-propanol production, the effects of enzyme source, acyl donor and solvent type, amount of added molecular sieves, acyl donor/alcohol molar ratio, and temperature on this enantioselective esterification reaction were investigated.
MATERIALS AND METHODS
Enzyme and Chemicals
Novozym 435 (Candida antacrtica lipase B) was obtained from Novozymes. Amona lipase PS (Pseudomonas cepacia) and Amona lipase AK (Pseudomonas fluorescens) were purchased from Aldrich (Germany). (R,S)-1-phenyl-1-propanol, lauric acid, myristic acid, palmitic acid, THF, heptane, toluene, isooctane, hexane, 2-propanol, and TLC sheets were purchased from Merck (Germany). The molecular sieve (4À) was purchased from Sigma (Germany). All chemicals were analytical grade.
Synthesis
(R,S)-1-phenyl-1-propanol (0.5 mmol) was dissolved in 3 ml of solvent. The desired amounts of acyl donors (lauric acid, myristic acid, and palmitic acid), molecular sieves (4À), and enzyme were added to the reaction medium. The esterification reaction was performed in a bottle with 10 ml on an orbital shaker at the desired stirring rate and temperature. The progress of the reaction was monitored by thin layer chromatography (TLC) and it was finished when the conversion reached about 50%. Under these conditions, S enantiomer of racemic 1-phenyl 1-propanol was obtained.
Analytics
The reaction progress monitored by TLC was stopped when the conversion reached to about 50%. After the solvent was evaporated with a rotary evaporator, the substrates were analyzed by HPLC using a Chiralcel-OB column. The mobile phase was hexane/2-propanol (97/3) with 0.8 ml/min flow rate at column temperature 30°C, and the compounds were detected at 254 nm with a diode array detector. The conversion and enantiomeric excess, (ee) of substrate were calculated by the equations x=[1−(CS+CR)/(CS0+CR0)]100 and ee=[(CS−CR)/(CS+CR)]100 (for CS>CR), respectively. Here, CS0 and CR0 are the initial substrate concentrations and CS and CR substrate concentrations at certain times of S and R form, respectively.
RESULTS AND DISCUSSION
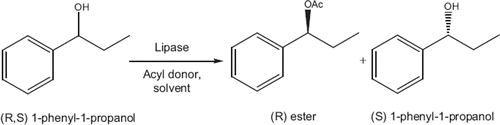
Lipase catalyzed enantioselective esterification of 1-phenyl-1-propanol was selected as a model system () for the biocatalytic resolution of secondary alcohols.
The enantioselectivity of the kinetic resolution of alcohol is influenced considerably by the source of enzyme, type of acyl donor, and solvent. First, the effects of enzyme source and solvent type on the kinetic resolution of (R,S)-1-phenyl-1-propanol were investigated. Three commercial lipases were screened for the kinetic resolution of racemic 1-phenyl-1-proponal with lauric acid and a molecular sieve at 40°C in toluene, hexane, heptane, and isooctane. The enzymes used were Novozym 435 (Candida antarctica lipase B), Amona lipase PS (Pseuodomonas cepacia), and Amona lipase AK (Pseudomonas fluorescens). All enzymes were in immobilized form. Immobilization of an enzyme is known to affect enzyme conformation, rigidity, and reactivity. In an organic solvent, lipase molecules usually aggregate, which reduces the activity, but when the lipases are immobilized they can be highly dispersed [Citation14]. In the enzymatic resolution of a specific chiral compound, the choice of enzyme is crucial for achieving the desired separation of enantiomers [Citation11]. As can be seen from , the highest ee (S) for substrate was obtained with Novozym 435 among all the enzymes. The reaction time is also very short and conversion is high for Novozym 435 compared to the other enzymes. Csajagi et al. [Citation2] and Irimescu et al. [Citation11] also obtained good results for the kinetic resolution of racemic alcohols using Candida antarctica lipase B.
Table 1. The effect of type of enzyme and solvent on the enantioselectivity of 1-phenyl 1-propanol (1-phenyl 1-propanol 167 mM, lauric acid 333 mM, enzyme 33.33 kg/m3, molecular sieve 33.33 kg/m3, solvent 3 mL, T=40°C, N = 150 rpm)
We investigated the influence of organic solvents with different log P values on the the enzymatic reaction for Novozym 435 and other enzymes. The influence of organic solvents on the enzymatic reaction for Novozym 435 using various fatty acids was also investigated. For this purpose, THF (log P 0.5), toluene (log P 2.5), hexane (log P 3.5), heptane (log P 4), and isooctane (log P 4.5) were used. The results are summarized in and . The maximum ee (S) obtained was 66% for 51% conversion with a 5.5 hour reaction time using toluene and Novozym 435. The minimum ee (S) was obtained for THF as 15% for 18% conversion with a 168 hours’ reaction time. These results show that the polar solvents have the lowest activity and enantioselectivity. The ranges of ee (S) values, conversion %, and reaction time were 66-51%, 51-40%, and 5.5-2.5 hours, respectively, for the other solvents. We preferred to use toluene the solvent with the highest enantioselectivity. The enzymes show higher activity with non-polar (log P>4) and mid-polar solvents (2<log P<4), whereas they have the lowest activity with polar solvents (log P<2) [Citation18]. Ducret et al. reported that log P did not correlate well with enzyme efficiency [Citation19]. In our study, there is no clear consensus about which log P of the solvents (2.5<log P<4.5) is related to the enantioselectivity.
Table 2. The effect of type of acyl donor and solvent on the enantioselectivity of 1-phenyl 1-propanol (1-phenyl 1-propanol 167 mM, acyl donor 333 mM, enzyme (Novozym 435) 33.33 kg/m3L, molecular sieve 33.33 kg/m3, solvent 3 mL, T=40°C, N=150 rpm)
The choice of acyl donor is also of great importance for the application of lipase in racemate solution. Fatty acids are the conventional acyl donors in the esterification reactions. They are preferred because they are more stable chemically, less toxic, cheaper, and more readily available than the other acylating reagents [Citation11]. The effect of acyl donor on enantioselectivity for esterification was investigated using lauric acid, myristic acid, and palmitic acid, which have different carbon numbers. As shown in , the carbon length of fatty acid from C12 to C16 did not affect ee (S). The maximum ee (S) obtained was 66% with lauric acid for toluene using Novozym 435. Suan and Sarmidi [Citation3] investigated the effect of chain length of fatty acids on the esterification reaction of (R,S)-1-phenyl ethanol. Lauric, myristic, palmitic and stearic acid as acylating agents were used. They reported that, although the carbon number of fatty acids affected the reaction rate, it did not influence the enantioselectivities of enzymes. Our results are similar to their findings that the enantioselectivity and reaction time were not influenced much by the achiral chain length of fatty acid in the esterification of 1-phenyl-1propanol. Lauric acid was considered the best acyl donor because of its good solubility in the organic solvent and high enantioselectivity.
The effect of the molar ratio of the acyl donor to alcohol on enantioselectivity was investigated. The experiments were performed in the range of 1/1-3/1 molar ratio of acyl donor to alcohol using lauric and myristic acid as acyl donors and toluene as solvent. Maximum ee (S) was obtained with the molar ratio of 1/1 for toluene and lauric acid as 77% (). ee (S) values decreased from 77% to 67% for lauric acid and from 72% to 65% for myristic acid with the increase in molar ratio of acyl donor to alcohol from 1/1 to 3/1. Conversion % also decreased from 51% to 41% for lauric acid. It was in the range of 45–44% for myristic acid. However, Suan and Sarmidi observed that the enantiomeric ratio of the enzymes was not affected by lauric acid concentration in the range of 25-250 mM [Citation3]. Our results show that substrate inhibition was observed when the concentration of lauric acid was over 167 mM (molar ratio of 1/1).
Table 3. The effect of acyl donor/alcohol mol ratio on the enantioselectivity of 1-phenyl 1-propanol (1-phenyl 1-propanol 167 mM, enzyme (Novozym 435) 33.33 kg/m3, molecular sieve 66.66 kg/m3, solvent (toluene) 3 mL, T=40°C, N= 150 rpm)
The water produced in the esterification reaction may deactivate the enzymes and decrease the the value of equilibrium conversion [Citation3]. Molecular sieves have been widely used to prevent the accumulation of water and improve the yield of enzyme catalyzed esterifications in organic media [Citation20]. At the same time, the structure and composition of a zeolite support influence the catalytic activity of an enzyme. The water produced was removed using a molecular sieve in this study. To investigate the effect of the molecular sieve on the enantioselectivity, 0–133.2 kg/m3 was added in to the reaction media. The effects of the molecular sieve amount on ee (S), conversion %, and reaction time are shown in using toluene as solvent and lauric acid and myristic acid as acyl donors, respectively. With the increase in molecular sieve amount from 0 to 133.2 kg/m3, ee (S) increased from 37% to 87% for lauric acid and from 36% to 83% for myristic acid. 133.2 kg/m3 molecular sieve was used, 87% ee (S) at 2.5 hours with 52% conversion was obtained for lauric acid, while 83% ee (S), and 54% conversion was obtained for myristic acid at 2.5 hours. At the same time, reaction time decreased with the increase in molecular sieve amount. This showed that preventing the water accumulation increased the reaction rate.
Table 4. The effect of amount of molecular sieve on the enantioselectivity of 1-phenyl 1-propanol (1-phenyl 1-propanol 167 mM, acyl donor (lauric acid) 167 mM, enzyme (Novozym 435) 33.33 kg/m3, solvent (toluene) 3 mL, T=40°C, N = 150 rpm)
It is known that the reaction temperature has a great effect on the enzymatic reaction. Temperature effects were investigated between 10 and 60°C using toluene as solvent and lauric acid as acyl donor (). When the temperature was increased, ee (S) increased. Probably, the activation energy of the reaction of the (R) enantiomer is higher than that of the reaction of the (S) enantiomer. Because of the reaction rate of the (R) enantiomer was faster than that of the (S) enantiomer, the maximum enantiomeric excess value was obtained at 50°C as 95% ee (S) with 55% conversion at 2.5 hours. At the same time, Novozym 435, which is an immobilized enzyme, has high stability at high temperature. The reaction time decreased from 6 to 2.5 hours with the increase in temperature depending on the increase in the reaction rate. The enantioselectivity and activity of Novozym 435 used in this study were affected by the change in temperature in contrast with Suan and Sarmidi's study [Citation3]. Csajagi et al. [Citation2] also investigated the influence of temperature on the enantiomer selectivity. They observed that enantiomer selectivity with increased with increasing temperature like the results in our study.
Table 5. The effect of temperature on the enantioselectivity of 1-phenyl 1-propanol (1-phenyl 1-propanol 167 mM, acyl donor (lauric acid) 167 mM, enzyme (Novozym 435) 33.33 kg/m3, molecular sieve 133.2 kg/m3, solvent (toluene) 3 mL, N = 150 rpm)
CONCLUSION
In order to improve the enantioselectivity of the kinetic resolution of racemic alcohol, screening of the enzyme is important. Three immobilized lipases were screened for the kinetic resolution of (R;S)-1-Phenyl-1-Propanol. The degree of activity and enantioselectivity differ widely for these enzymes. Novozym 435 exhibited the highest performance for the resolution among the enzymes. The choice of solvent and acyl donor is also of great importance. Enzyme activity is affected by the hydrophobicity of the solvent and reactants, solvent polarity, and water miscibility. High enantioselectivity was obtained for toluene. Probably, it affected the enzyme conformation. Increasing the molar ratio of the acyl donor to alcohol from 1/1 to3/1, decreased the enantioselectivity. When the molecular sieve amount and temperature were increased ee (S) increased and the reaction time decreased for about 50% conversion. Molecular sieves prevented the accumulation of water and improved the yield of enzyme catalyzed esterifications. At the same time, the structure and composition of a zeolite support influence on the catalytic activity of an enzyme. When the temperature was increased enantioselectivity and reaction rate increased. The maximum value was obtained at 50°C using 133.2 kg/m3 molecular sieve as 95% ee (S), and 55% conversion at 2.5 hours. As a result, enzymatic esterification using a molecular sieve has good potential applicability for kinetic resolution of secondary alcohols.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Hayos, P., Buthe, A., Ansorge-Schumacer, M.B., Sinisterra, J. V., Alcantara, A. R.. (2008) Highly efficient one pot dynamic kinetic resolution of benzoins with entrapped P.stutzeri lipase. Journal of Molecular Catalysis B: Enzymatic, 52:133–139.
- Csajagi, C., Szatzker, G., Töke, E. R., Ürge, L., Darvas, F., Poppe, L. (2008). Enantiomer selective acylation of racemic alcohols by lipases in continiuous-flow bioreactors. Tetrahedron-Asymmetry19:237–246.
- Suan, C., Sarmidi, M.R. (2004). Immobilized lipase-catalysed resolution of (R,S)-1-phenyl ethanol in recirculated packed bed reactor. J. Mol. Catal. B:Enzymatic, 28:111–119.
- Berglund, P. (2001). Controlling lipase enantioselectivity for organic synthesis. Biomol. Eng., 18:13–22.
- Mehmetoğlu, Ü., Bayraktar, E., Babaarslan, Ç. (2007). Production of enantiomerically pure pharmaceutical compound using biocatalyst. In: Enzyme Mixture and Complex Biosynthesis, Bhattacharya, S.K. Austin: Landes Bioscience, 65–73.
- Reetz, M. T.. (2002) Lipases as practical biocatalysts. Curr. Opin.Chem.Biol., 6:145–150.
- Theil, F. (2000). Enhancement of selectivity of reactivity of lipases by additives. Tetrahedron, 56:2905–2919.
- Bakker, M., Spruijt, A. S., Van Rantwijk, F., and Sheldon, R. A.. (2000) Highly enantioselective aminoacylae-catalyzed transesterification of secondary alcohols. Tetrahedron-Asymmetry, 11:1801–1808.
- Hirose, K., Naka, H., Yano, M. (2000). Improvement of enantioselectivity in kinetics resolution of a primary alcohol through lipase-catalysed transesterification by using a chiral acyl donor. Tetrahedron-Asymmetry, 11:1199–1210.
- Kiss, V., Egri, G., Bailent, J., Ling, I., Barkoczi, J., Fogassy, E. (2006). Kinetic and chemical resolution of different 1-phenyl-2-propanol derivatives. Tetrahedron-Asymmetry, 17:2220–2234.
- Irimescu, R., Saito, T., Kato, K. (2004). Enzymatic kinetic resolution of primary alcohols by direct esterificationin solvent free system. Journal of Molecular Catalysis B: Enzymatic, 27:69–73.
- Holmquist, M., Haeffner, F., Norin, T., Hult, K. (1996). A structural basis for enantioselective inhibition of Candida rugosa lipase by long-chain aliphatic alcohols. Protein Sci., 5:83–88.
- Ottoson, J., Hult, K. (2001). The influence of acyl chain length on enantioselectivity of Candida antactica lipase B and its thermodynamic components in kinetic resolution of sec-alcohols. Journal of Molecular Catalysis B: Enzymatic, 11:1025–1028.
- Frings, Koch, K., M., Hatmeier, W. (1999). Kinetic resolution of 1-phenyl ethanol with high enantioselectivitiy with native and immobilized lipase in organic solvents. Enzyme Microb. Technol., 25: 303–309.
- Sakai, T. (2004). Low-temperature method for a dramatic improvement in enantioselectivity in lipase- catalyzed reactions. Tetrahedron-Asymmetry, 15:2749–2756.
- Tewari, Y. B., Vanderah, D. J., Rozzell, J. D.. (2003) Thermodynamics of the lipase catalyzed transesterification of 1-phenyl-1-alkonols and butyl acetate in organic solvents. Journal of Molecular Catalysis B: Enzymatic, 21:123–131.
- Bachu, P., Gibson, J. S., Sperry, J, Brimble, M. A.. (2007) The influence of microwave irradiation on lipase catalysed kinetic resolution of racemic secondary alcohols. Tetrahedron: Asymmetry, 18:1618–1624.
- Huang, W., Xia, Y.M., Gao, H., Fang, Y.J., Wang, Y., Fang, Y. (2005). Enzymatic esterification between n-alcohol and n-caprylic acid in non-aques medium under microwave irradiation. Journal of Mol Catal B:Enzymatic, 35:113–116.
- Ducret, Trani, A., M., Lortie, R. (1998). Lipase-catalyzed enantioselective esterification of ibuprofen in organic solvents under controlled water activity. Enzyme Microb. Technol., 22:212–216.
- Fortes, Partridge, N., J., Halling, P.J., Barreiros, S. (2002). Zeolite molecular sieves have dramatic acid-base effects on enzymes in nonaqueous media. Biotechnology and Bioengineering, 77:296–305.