Abstract
This study was designed to investigate the influence of polymerized human placenta hemoglobin (PolyPHb) pretreatment on ischemia/reperfusion (I/R) injury-induced myocardial apoptosis. Isolated Sprague-Dawley rat hearts were perfused by Langendorff model. After basal perfusion, hearts were arrested by St.Thomas’ solution (STS) with (Pre-HBOC group) or without PolyPHb (STS group), then subjected to I/R injury. Our results showed that PolyPHb pretreatment greatly reduced the TUNEL-positive myocardial cells and decreased the caspase-3 activity and cleavage, which was further confirmed by the linear regression analysis. Therefore, PolyPHb pretreatment was beneficial to attenuation of myocardial apoptosis and provided protection to the heart against I/R injury.
INTRODUCTION
Apoptosis is an active form of cell suicide affecting cardiac myocytes during ischemia/reperfusion (I/R) injury [Citation1–3]. Therefore, reduction of myocardial apoptosis is believed to be a positive way to attenuating cardiac I/R injury. Polymerized human placenta hemoglobin (PolyPHb), one type of hemoglobin-based oxygen carrier (HBOC), was initially developed by the research group of Professor Chengmin Yang to treat patients suffering from trauma and hemorrhagic shock [Citation4,Citation5]. However, our previous studies revealed that PolyPHb might also be a promising protective agent for I/R heart [Citation6–8]. In our latest study, we provided distinct evidence that PolyPHb pretreatment was helpful against cardiac I/R injury by improving cardiac function and reducing myocardial infarction. To further prove the cardioprotective effect of PolyPHb, we designed this study to investigate the influence of PolyPHb pretreatment on myocardial apoptosis of isolated rat hearts after warm ischemia and reperfusion.
MATERIALS AND METHODS
The present study was performed in adherence with the Guidelines on the Use of Laboratory Animals published by the National Institutes of Health and approved by the Animal Care and Use Committees in Sichuan University.
PolyPHb Preparation
PolyPHb was prepared as we previously described [Citation4,Citation5]. Briefly, hemoglobin from fresh human placenta blood (donated by Tianjin Union Stem Cell and Genetic Engineering Ltd., Tianjin, China) was purified and viral inactived by heat treatment, then intra- and inter-molecularly cross-linking were performed by using pyridoxal phosphate (PLP) and glutaraldehyde (GDA), respectively. After that, ultrafiltration and molecular sieve chromatography were performed to harvest PolyPHb with molecular weight range from 64 KD to 600 KD. Before use, the prepared PolyPHb solution was added into St.Thomas’ solution (STS) to a final concentration of 0.1 gHb/dL, and bubbled with 100% oxygen gas for 10 mins.
Experimental Proposal
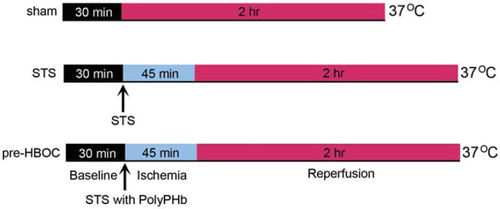
Thirty male Sprague-Dawley rats, weighing 250–300g, were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg) and heparin (500 IU). The hearts were quickly excised and perfused on a Langendorff apparatus with Krebs-Henseleit buffer (KHB: 120.0 mM NaCl, 4.5 mM KCl, 20.0 mM NaHCO3, 1.2 mM KH2PO4, 1.2 mM MgCl2, 2.5 mM CaCl2 and 10.0 mM glucose, pH 7.4, 37°C) at a constant pressure of 100 cmH2O. A thin-wall latex balloon was inserted into the left ventricle (LV) through the left atrium to continuously monitor the LV function (ADInstruments Pty Ltd., Bella Vista, NSW, Australia. After 30 mins of basal perfusion, hearts were randomly assigned to sham, STS, and pre-HBOC groups. In the pre-HBOC and STS groups, the hearts were arrested by St.Thomas’ solution (STS) with or without 0.1 gHb/dL PolyPHb (10mL/min × 2min), respectively. Then, warm ischemia (37°C) for 45 mins and KHB reperfusion for 2 hrs were performed (). The sham group hearts were perfused by KHB for 2.5 hrs without ischemia.
Figure 1. The experimental protocol of this study. After 30 mins of basal perfusion, the pre-HBOCs and STS groups’ hearts were arrested by STS with or without 0.1 gHb/dL PolyPHb, then subjected to 45 mins of ischemia at 37°C and 2 hrs of reperfusion. Hearts perfused with Krebs-Henseleit buffer for 2.5 hrs without ischemia were used as sham control. STS: St.Thomas’ solution.
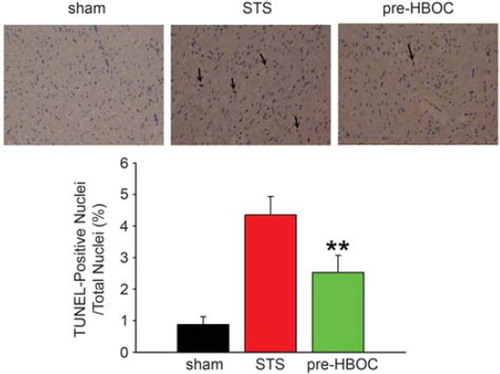
TUNEL-Positive Myocardial Cells Determination
Extent of myocardial apoptosis was determined using terminal dUTP nick-labeling (TUNEL) as described previously [Citation8]. Briefly, the LV tissues were immediately fixed in 4% paraformaldehyde solution for 24 hr and embedded in paraffin. Then, the paraffin sections were prepared and processed for the TUNEL technique (Roche, Indianapolis, IN). The extent of myocardial apoptosis was expressed as the percent of the TUNEL-positive to the total myocardial cells.
Caspase-3 Activity Measurement
The cardiac caspase-3 activity, another indicator of apoptosis, was measured from the LV tissue homogenate using a Caspase-Glo 3/7 assay kit (Promega, Madison, WI).
Western Blot Assay
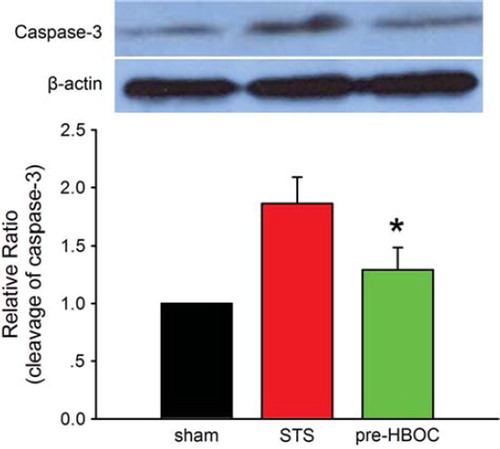
The caspase-3 cleavage was determined by western blot assay as we described previously [Citation6]. The protein concentration of LV tissue homogenate was determined by the BCA method (Pierce, Rockford, IL). Samples containing 20 μg protein were separated with SDS-PAGE, and transferred to a nitrocellulose membrane. Primary antibodies included caspase-3 (Santa Cruz Inc., Santa Cruz, CA) and b-actin (Abcam, Cambridge, MA). The membrane was then incubated with a HRP-conjugated anti-mouse (1:5000, Pierce, Rockford, IL) for 1 hr. A Supersignal chemiluminescence detection kit (Pierce, Rockford, IL) was used to quantify the protein of interest. Immunoblot was visualized with a Kodak X-ray Processor 102 (Eastman Kodak, Rochester, NY), and analyzed with Quantity One software (Bio-Rad Laboratories, Hercules, CA).
Statistical Analysis
All values in this study were presented as mean ± SD and analyzed by one-way ANOVA followed by LSD correction for post hoc t test or linear regression analysis (SPSS 13.0 software). P values < 0.05 were considered statistically significant.
RESULTS
PolyPHb Pretreatment Reduced Myocardial Apoptosis
After 45-min warm ischemia and 2-hr reperfusion, significantly increased TUNEL-positive myocardial cells were observed in the STS group (4.36 ± 0.58% vs. 0.87 ± 0.25% in the sham group, P< 0.001, ). However, in the pre-HBOC group, the apoptotic myocardial cells were remarkably reduced when compared with the STS group (2.53 ± 0.54%, P<0.01).
PolyPHb Pretreatment Inhibited Caspase-3 Activity and Cleavage
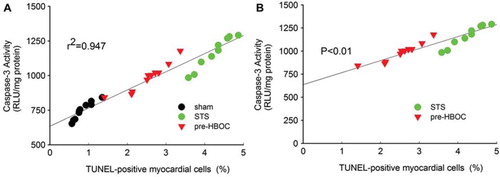
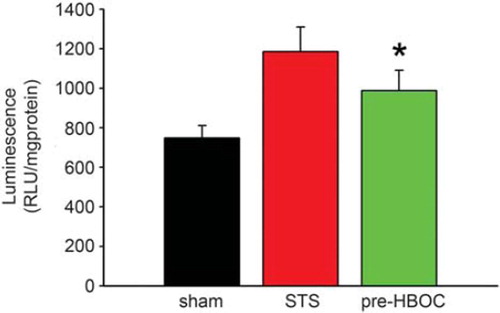
As shown in , the sham group hearts exhibited minimal caspase-3 activity (748.59 ± 62.33 RLU/mg protein). After I/R injury, the caspase-3 activity was greatly increased (1185.95±124.35 RLU/mg protein in the STS group, P < 0.01 vs. the sham group). PolyPHb pretreatment significantly reduced such an increase in the caspase-3 activity (987.94±102.85 RLU/mg protein, P < 0.05 vs. the STS group). As the active form of caspase-3, the caspase-3 cleavage (p17 subunit) plays an important role in apoptosis execution. In this study, western blot assay indicated that the cleavage of caspase-3 was also significantly depressed by PolyPHb pretreatment when compared to the STS group (P< 0.05, ). The results of linear regression analysis showed that: (1) there was a great correlation between myocardial apoptosis and caspase-3 activity (r2=0.947, ) (2) there was a significant difference between the STS group and pre-HBOC group (P < 0.01, ).
Figure 3. The caspase-3 activity of three group hearts. Values are presented as mean ± SD (n = 10). *P < 0.05 vs. the STS group.
DISCUSSION
As we know, HBOC was initially developed as a blood substitute [Citation9, Citation10]. However, there were several complications associated with systemic administration of HBOC, such as hypertension, renal toxicity, coronary and cerebral vasospasm, gastrointestinal side effects, chest and abdominal pain, which have not been totally solved at present [Citation11–14]. Therefore, in addition to improving the quality of HBOC itself, we have started to explore the alternative clinical uses of HBOC from several years ago [Citation6–8,Citation15].
The present study demonstrated that PolyPHb pretreatment significantly reduced myocardial apoptosis after warm ischemia and reperfusion, which was evidenced by the greatly reduced TUNEL-positive myocardial cells and limited caspase-3 activity and cleavage. The result of linear regression analysis provided further evidence that PolyPHb pretreatment was beneficial to decreasing myocardial apoptosis. Considering the promising oxygen transporting capacity and optimal molecular weight, we attributed the anti-apoptotic effect of PolyPHb to the myocardium oxygen preload before warm ischemia. In the previous study, we found that the “hyperoxic preconditioning” from PolyPHb pretreatment, as “ischemic preconditioning,” also provided protection to the heart against I/R injury. This study confirmed our previous results and indicated that the cardioprotective effect of PolyPHb pretreatment was associated with the attenuation of myocardial apoptosis. The results of the present study provided additional evidence that PolyPHb was a promising agent for cardioprotection.
In conclusion, using a Langendorff isolated heart model we proved that the PolyPHb pretreatment was beneficial to reduction of myocardial apoptosis, thus providing protective effect to an isolated rat heart against I/R injury.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Moens, A. L., Claeys, M. J., Timmermans, J. P., Vrints, C. J. (2005). International Journal of Cardiology 100:179–190.
- Ferdinandy, P., Schulz, R., Baxter, G. F. (2007). Pharmacological Reviews 59:418–458.
- Scarabelli, T. M., Stephanou, A., Pasini, E., Comini, L., Raddino, R., Knight, R. A., Latchman, D. S. (2002). Circ Res 90:745–748.
- Li, T., Yu, R., Zhang, H. H., Wang, H., Liang, W. G., Yang, X. M., Yang, C. M. (2006). Artif Cells Blood Substit Immobil Biotechnol 34:175–188.
- Li, T., Zhang, H., Wang, H., Yu, R., Yang, C. (2006). Journal of Biomedical Engineering 23:640–644.
- Li, T., Li, J., Liu, J., Zhang, P., Wu, W., Zhou, R., Li, G., Zhang, W., Yi, M., Huang, H. (2009). Free Radical Biology and Medicine 46:397–405.
- Li, T., Liu, J., Yang, Q., Wu, W., Zhang, P., Yang, J., Li, J., Zhang, W., Yang, C. (2009). Artif Cells Blood Substit Immobil Biotechnol 37:48–52.
- Li, T., Zhang, P., Liu, J., Zhou, R., Li, Q., You, Z., Dian, K. (2010). Free Radical Biology and Medicine 48:1079–1089.
- Fergusson, D. A., McIntyre, L. (2008). JAMA 299:2324–2326.
- D'Agnillo, F., Chang, T. M. (1998). Nat Biotechnol 16:667–671.
- Burmeister, M. A., Rempf, C., Standl, T. G., Rehberg, S., Bartsch-Zwemke, S., Krause, T., Tuszynski, S., Gottschalk, A., Schulte am Esch, J. (2005). Br J Anaesth 95:737–745.
- Jahr, J. S., Nesargi, S. B., Lewis, K., Johnson, C. (2002). American Journal of Therapeutics 9:437–443.
- Winslow, R. M. (2003). J Intern Med 253:508–517.
- Natanson, C., Kern, S. J., Lurie, P., Banks, S. M., Wolfe, S. M. (2008). JAMA 299:2304–2312.
- Wu, W., Yang, Q., Li, T., Zhang, P., Zhou, R., Yang, C. (2009). Artif Cells Blood Substit Immobil Biotechnol 37:163–165.