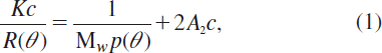Abstract
The gelatin plasma substitute is often polydisperse and heterogenous, making it difficult to determine the elimination rate and half-life in the body. In this study, one method was developed based on quantitative determination of hydroxyproline derivatives. Two plasma substitutes were prepared by succinylation and genipin-crosslinking, respectively. After transfusion, the blood samples were hydrolyzed and derivatized, and then analyzed by HPLC. A two-phase exponential association equation was used for fitting the time-concentration curves. The results indicated that this method could be used for quantitative determination of gelatin in blood, and the pharmacokinetic parameters such as elimination rate and half-life.
INTRODUCTION
Plasma substitutes are solutions of colloids or crystalloids administered intravenously to restore circulatory blood volumes in medical emergencies, resuscitation, etc. The requirements for a qualified plasma substitute are related to its physico-chemical properties, biological properties and pharmacokinetics [Citation1–3]. The duration of the oncotic effect is one of the most important pharmacokinetic parameters reflecting the rate of absorption, degradation, and/or renal excretion of plasma substitute in body [Citation4–7]. Thus, determination of intravascular elimination rate and half-life in vivo is crucial to evaluate the clinical efficacy of a plasma substitute, especially for predictable controlling of the duration of the oncotic effect.
Gelatin-based plasma substitutes have been intensively investigated for clinical treatment of patients requiring an increase in circulating blood volume [Citation8,Citation9]. However, it is difficult to directly determine the gelatin derivatives in blood circulation system since gelatin is composed of complex polypeptides. Many methods have been established to determine the collagen contents in biological specimens [Citation10–13]. However, due to the polydispersity and heterogeneity of gelatin, and interference from blood components, most of these methods were not suitable for quantitatively determination of gelatin plasma substitutes in blood. Radioactive labeling offers a possibility for the investigation of retention and dispersal of gelatin plasma substitute [Citation8,Citation14], but the process is complicated and needs special protective measures. Thus, scientists are contemplating more explicit methods concerning the elimination of gelatin-based plasma substitutes. Gelatin is composed of a mixture of polypeptides obtained by partial hydrolysis of collagen. The amino acid sequence of collagen often follows the pattern Gly-X-Y. Hydroxyproline is one major amino acid component of collagen and is rarely found in other proteins [Citation15]. Numerous methods for hydroxyproline determination have been developed and modified over the past fifty years, but scientists are more interested in various pathological conditions based on hydroxyproline level [Citation16–18]. Considering the comparatively large infused volume and high hydroxyproline content in gelatin, the determination of hydroxyproline could be utilized for the assay of gelatin plasma substitutes in blood.
Gelatins generally show many disadvantages if they are directly used as a plasma substitute. Gelatins have to be chemically modified or crosslinked to improve their chemical and physical properties and meet the needs of clinical applications [Citation19]. As a traditional and widely used plasma substitute, succinylated gelatin was prepared by chemical modification with succinic anhydride [Citation20,Citation21]. Due to the changed ratio of free amino to carboxylic group, the gelatin isoeletric point and gelability were altered, and then the colloid osmotic function could be improved. Some crosslinking agents can also be used for improving the gelatin properties, such as diisocyanate and glyoxal. The branched chains allow an increase in molecular weight and therefore improve the colloid osmotic pressure and intravascular retention, with no significant increase in viscosity [Citation1]. Genipin was an aglycone derived from geniposide present in the fruit of Gardenia jasminoides. As a naturally occurring and biodegradable molecule with low cytotoxicity and antigenicity, genipin has recently been employed as a crosslinking agent in many biological aspects [Citation22]. It was found that genipin also showed pharmacological effects such as anti-inflammatory activity, protective activity against oxidative damage, and fibrolytic activity [Citation23–25]. Recent explorations into the use of genipin-crosslinked gelatin, such as bioadhesive, wound dressing, and bone substitute, showed that genipin might act as an alternative crosslinking agent for gelatin plasma substitutes [Citation26–29]. In addition, the anti-inflammatory activity of genipin would be helpful for decreasing the inflammatory response during transfusion and postoperative recovery.
In this study, two gelatin plasma substitutes were prepared by using succinic anhydride and genipin as modifying/crosslinking agents. Their capacity of blood volume expanding and safety were investigated. Hydroxyproline derivatized by 2, 4-Dinitro-1-Fluorobenzene (DNFB) was used as a marker for the quantitative determination of intravascular elimination rates and the in vivo half-live of gelatin plasma substitutes.
MATERIALS AND METHODS
Instruments and Chemicals
Agilent 1100 liquid chromatography combining a photodiode array detector (Hewlett Packard, Palo Alto, CA, USA). Dawn EOS multi-angle laser light scattering detector and Optilab DSP interferometric refractometer (Wyatt Technology, Santa Barbara, California, USA). DNFB was purchased from Sigma-Aldrich (St. Louis, MO, USA). Genipin was purchased from Wako Pure Chemical Industries (Osaka, Japan). Trypsin (sequencing grade) was purchased from Promega (Madison, WI, USA). All other reagents were of analytical grade.
Animals
New Zealand white rabbits (male, 2.3–2.8 kg) were obtained from the Animal Center of Peking University Health Science Center. They were housed in cages at a temperature of 24 ± 1°C with free access to food and water. Animals were fasted for 24 h with water ad libitum before blood drawing and infusion of the gelatin plasma substitutes.
Preparation of Succinylated Gelatin
Bovine gelatin was heat-degraded and purified to a number average molecular weight of approximately 28,000. Gelatin solution with a concentration of 6% (w/v) was incubated at 37°C and pH was adjusted to 9.0 with NaOH. Succinic anhydride (1/10 weight of gelatin) was gradually added into the gelatin solution with continuous stirring, and pH was maintained within 8.5–10.0 during the reaction. The succinylation was regarded as finished when the pH showed no further changes. The process took approximately 1 h. The solution was then acidified with HCl to pH 2.7 followed by centrifugation. The precipitates were redissolved in pure water and NaCl was gradually added to approximate 30% (w/v). The solution was then centrifuged. The precipitate was dried and redissolved with a final concentration of 4% (w/v).
Preparation of Genipin-crosslinked Gelatin
Bovine gelatin was hydrolyzed with trypsin and purified to a number average molecular weight of approximately 15,000. Gelatin solution with a concentration of 6% (w/v) was incubated at 55°C and pH was adjusted to 7.4 with NaOH. Then genipin (0.2% weight of gelatin) was gradually added into the gelatin solution with continuous stirring. The pH was maintained within 7.2–7.6 during the reaction process. This process took approximately 8 h. The solution was then acidified with HCl to pH 4.5 and then cold ethanol was gradually added to 50% (v/v). After that the solution was centrifuged and the precipitate was removed. Ethanol was gradually added into the supernatant to 80% (v/v). The precipitate was collected, dried, and redissolved with a final concentration of 4% (w/v).
0.9% NaCl (w/v) also included in both gelatin solutions, and solutions were prefiltered through a 0.22 μm polyethersulfone membrane prior to use.
HPLC Analysis of Amino Acid Derivatives
The HPLC analysis was performed on a Zorbax SB C18 column (4.6 mm × 250 mm, 5 μm). Solvent A was 0.05 M sodium acetate in water and solvent B was acetonitrile/H2O (1:1, v/v). The optimized linear gradient was: 0–5 min, 30%–55% B; 15–25 min, 55%–100% B. Detection wavelength: 360 nm; flow rate: 1.0 ml/min; column temperature: 25°C
In Vitro Stability of Hydroxyproline during Hydrolyzation
Hydroxyproline was dissolved in 1 ml of 6 M HCl with concentrations from 0.5 μg/ml to 500 μg/ml, and thenhydrolyzed at 110°C in sealed glass ampoules for 12 h. The solutions were neutralized to pH 7.0 with NaOH and then water was added to a constant volume of 10 ml. 100 μl volumes were withdrawn from each and mixed with 200 μl of 0.5 M NaHCO3 buffer and 10 μl of DNFB. The derivatization reaction was carried out at 60°C for 1 h, then 1 ml of 0.1 M NaH2PO4 (pH 7.0) was added. The samples were subsequently filtrated and analyzed with HPLC with an injection volume of 10 μl. To investigate the effect of hydrolysis on hydroxyproline loss, hydroxyproline solutions of the same concentration without hydrolysis were derivatized directly with DNFB, and were used as references.
In Vitro Assay of Blood/Substitute Mixtures
0.5 ml of blood samples were mixed with 0, 0.01, 0.05, 0.1, 0.2 and 0.5 ml genipin crosslinked gelatin solution, respectively, and water was then added to a final volume of 1 ml. The relative content (v/v) of plasma substitute was 0%, 1.96%, 9.10%, 16.67%, 28.6% and 50%, respectively. 100 μl of sample was taken from each mixture, and then hydrolyzed and derivatized before HPLC analysis.
Blood Drawing
Six rabbits were anesthetized with sodium pentobarbital (30 mg/kg). The right carotid artery of the rabbits was cannulated for blood drawing. 30% total blood volume (total blood accounted for approximately 5% body weight) was withdrawn via carotid artery and the same volumes of plasma substitute were then infused via internal jugular vein. Three of the rabbits were infused with succinylated gelatin, and the other three were infused with genipin crosslinked gelatin. 0.2 ml of blood samples were withdrawn from the auricular veins at different time within 50 hours postinjection.
Determination of Relative Content of Hydroxyproline in Blood
100 μl of blood samples were hydrolyzed and then amino acid derivatization was carried out. 10 μl of sample solution was injected for HPLC analysis.
RESULTS AND DISCUSSION
Characteristics of Succinylated and Genipin-crosslinked Gelatin
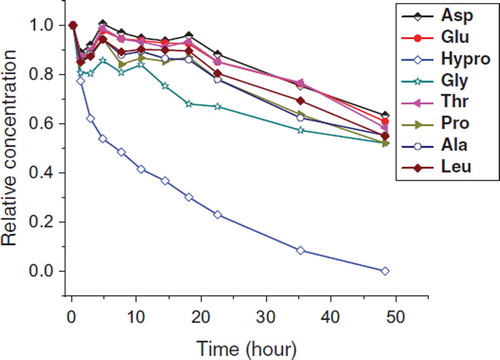
The physicochemical characteristics of two different kinds of plasma substitutes are listed in . The number average molecular weight and molecular weight distribution of both gelatin plasma substitutes were determined by size exclusion chromatography coupled with multi-angle laser light scattering (SEC-MALLS) [Citation31,Citation32]. As shown in , RI signals of both two gelatin plasma substitutes were close to a smooth normal distribution, which would be helpful for rapid blood volume expansion and smooth maintenance of colloid osmotic pressure (COP) after transfusion [Citation1]. MALLS is a straight method to determine molecular weights and sizes without assumptions. In SEC-MALLS, average molecular weight is calculated according to equation (1) [Citation35,Citation36].
Figure 1. SEC-RI-MALLS detection of gelatin plasma substitutes. A: Succinylated gelatin; B: Genipin crosslinked gelatin.
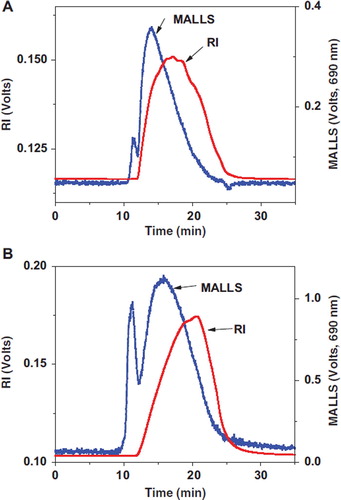
Table 1. Physicochemical characteristics of gelatin plasma substitutes
where K is the polymer constant for a particular solution, c is the concentration of eluted fraction, R (θ) is the excess Rayleigh ratio, P (θ) is the particle scattering function, and A is the second virial coefficient. By taking the measurements at multiple angles, the molecular weight of each eluted fraction could be determined by extrapolating Kc/R(θ) to zero angle (Zimm method). Different from the RI signals, a sharp peak with retention time from 11 to 12 min was observed on each MALLS chromatogram. The peak fraction eluted in void volume contained gelatin peptides with molecular mass exceeding the valid separation range of the column used. Laser signal is sensitive to large molecules according to Equation 1. As can be seen from , some gelatin peptides with high molecular weight and low content, which could not be detected by UV or RI detector, can be detected sensitively by MALLS. These low abundance components had been proved not causing in vivo side effect in animal experiments.
Hydroxyproline Analysis for Gelatin Quantification in Blood
In vitro stability of hydroxyproline during hydrolyzation. Hydroxylation of proline is a kind of post-translational modification. Hydroxyproline is abundant in collagen and lacking in almost all other proteins. Therefore it could be used as a specific maker for the quantitative determination of gelatin plasma substitute in blood. However, blood samples must be hydrolyzed completely to release free amino acids for DNFB derivatization before HPLC analysis. The stability of hydroxyproline during hydrolysis should be investigated. Hydroxyproline solutions with different concentrations were prepared. One group was hydrolyzed before derivatization; the other duplicated group was directly derivatized. Compared to the sample in which hydroxyproline was not hydrolyzed, the hydrolyzation group remained an average 97.8% of hydroxyproline recovery with a RSD of 1.2% (n=6). This result indicates that the intense hydrolysis condition shows no obvious influence on content of hydroxyproline. The limit of quantification (S/N > 10) was 0.5 μg/ml for hydroxyproline derivatives, and the linearity was ranging from 0.5 μg/ml to 500 μg/ml. This means that the gelatin plasma substitutes (4%, w/v) can be quantitated after as high as 1000 times of dilution (the hydroxyproline content in gelatin is determined as 12.5%).
In vitro analysis of substitute/blood mixtures. In order to evaluate the possible interference of blood components to hydrolyzation, derivatization and HPLC separation, gelatin plasma substitutes were mixed in vitro with the original rabbit's blood. The mixtures were then hydrolyzed, derivatized, and analyzed by HPLC.
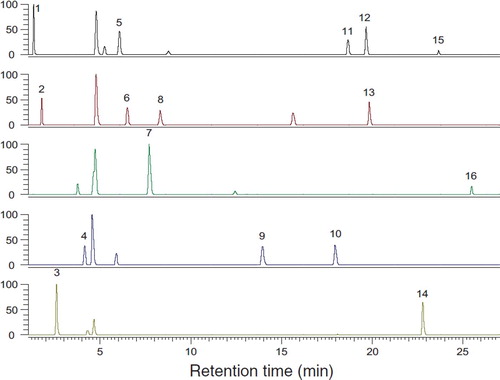
Sixteen standard amino acids were divided into five groups for convenient peak assignment and each group contained 2–5 amino acids. Each group was derivatized with DNFB and then analyzed by HPLC. As shown in , amino acid peaks in all the groups were successfully separated and the retention time of each amino acid in chromatograms was assigned.
Figure 2. Chromatograms of different free amino acids derivatives. 1: Aspartic acid; 2: Glutamic acid; 3: Hydroxyproline; 4: Serine; 5: Glycine; 6: Threonine; 7: Proline; 8: Alanine; 9: Valine; 10: Isoleucine; 11: Leucine; 12: Methionine; 13: Phenylalanine; 14: Histidine; 15: Lysine; 16: Tryptophane.
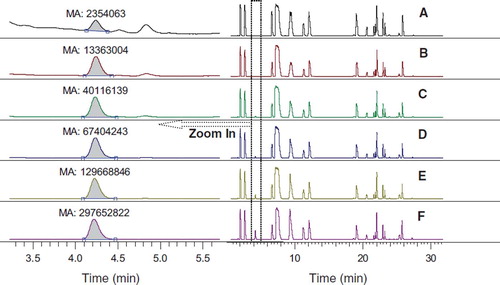
The ratio of gelatin plasma substitute would be below 40% in blood after transfusion. Therefore, 6 gelatin solutions (4% w/v) were mixed separately with original rabbit blood in vitro, resulting in a final gelatin solution ratio varying from 0 to 50%. Mixtures were then hydrolyzed and derivatized by DNFB before HPLC determination. As shown in , almost all amino acids were separated successfully, especially for hydroxyproline. The concentrations of hydroxyproline were all included in the quantification linearity range from 0.5 μg/ml to 500 μg/ml. The relative content of gelatin in gelatin/blood mixture had a good linearity relationship with peak area of hydroxyproline derivatives. The correlation coefficients (R2) were above 0.99. The x-intercept was 0.004, and this value represented the relative content of hydroxyproline in original blood compared with 4% (w/v) gelatin plasma substitute (set as 100%).
Figure 3. Chromatograms of hydroxyproline derivatives from blood/gelatin mixtures. Blood/gelatin ratio: A: 100:0; B: 100:2; C: 100:10; D: 100:20; E: 100:50; F: 100:100.
In vivo analysis of blood samples from hemorrhagic rabbits. All blood samples withdrawn from experimental rabbits after 30% of blood drawing and gelatin infusion were directly hydrolyzed and then derivatized for hydroxyproline determination. The relative content of hydroxyproline in blood taken at 5 min after the transfusion was set as 100%. The change of remained gelatin plasma substitute in blood with time is shown in . The pharmacokinetic parameters were obtained by fitting the data to a two-phase exponential association equation as follows.
Figure 4. Relative content change of gelatin plasma substitutes in vivo after transfusion. A: Succinylated gelatin; B: Genipin crosslinking gelatin.
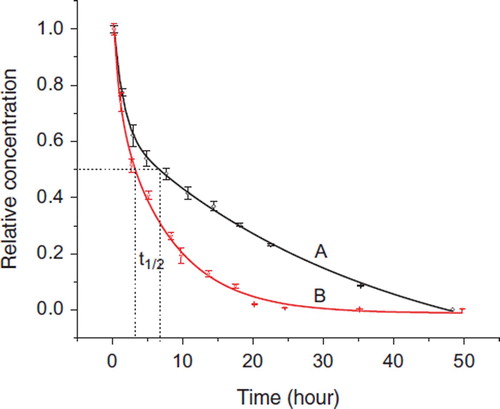
where y0 is the relative content of hydroxyproline in original blood, and determined as 0.004. Similar to the two compartment model, Ae−αt presents the distribution phase, Be−βt represents the elimination phase, α and β are hybrid constants controlling the rates of distribution and elimination, respectively [37]. The pharmacokinetic parameters of the succinylated and genipin-crosslinked gelatins are listed in . As shown in , both curves were well fitted to the above equation with R values above 0.99. The half-life of the succinylated and genipin-crosslinked gelatin were calculated as 6.7±0.2 h and 3.3±0.2 h (n=3), respectively. 88% succinylated gelatin and 96% genipin-crosslinked gelatin were eliminated from blood after 24 h of transfusion. 3 and 4 transit compartments were also tried to fit the data, and the correlations were poor compared with the 2 transit compartments model.
Table 2. Dynamic modeling parameters of succinylated and genipin crosslinked gelatina
Besides hydroxyproline, other amino acids that are also abundant in collagen were also tried as amino acid marker for analysis. According to the data obtained from the in vitro gelatin/blood mixture experiment, other amino acids also had good linearity relationships between the peak area of corresponding amino acids derivatives and gelatin relative content in mixture, but these amino acids would not be a good marker because their contents are unstable in vivo. After blood drawing and gelatin transfusion, the blood contained the original blood and infused gelatin. Blood regeneration and the compensatory mechanism would be accelerated, which would continuously increase various proteins in blood. As a result, most of the amino acid contents would be affected and the results will be inaccurate. However, hydroxyproline was almost not affected as this amino acid is abundant in gelatin but almost absent in other blood proteins. shows the relative content of eight major amino acids vs. time. The contents of the amino acids decreased in irregular patterns, especially in the early stage within 18 hours, except hydroxyproline. It is notable that glycine, which accounts for approximately 30% of the amino acids in collagen, also showed an irregular decrease, indicating that glycine of blood origin significantly interfered with the determination of glycine from gelatin.
CONCLUSION
The degradation and excretion of plasma substitutes in body, especially the pharmacokinetic parameters such as the elimination rate and half-life in blood, are important for their evaluation of clinical efficacy. Finding some specific marks in those polydisperse plasma substitute solutions to quantitate that remaining in the blood is a simple and direct way to this aim. In this research, the method based on detection of hydroxyproline derivatives was successfully used for the determination of modified and crosslinked gelatin remained in blood. Pharmacokinetics of gelatin plasma substitutes can be described by two-phase exponential association equation and their elimination rate and half-life can be calculated. There are many kinds of derivative reagents such as DNFB, dabsyl chloride, fluorescamine, and phenylisothiocyanate [Citation36]. DNFB was adopted since it showed a good sensitivity and stability in this research. Other derivative reagents may also be used for this purpose after similar investigation. These methods would be also used for determining the collagen contents in biological specimens.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Liberati, A., Moja, L., Moschetti, I., Gensini, G.F., Gusinu, R. (2006). Human albumin solution for resuscitation and volume expansion in critically ill patients. Intern Emerg Med 1:243–245.
- Booth, C., Highley, D. (2010). Crystalloids, colloids, blood, blood products and blood substitutes. Anaesth Intens Care, 11:50–55.
- Mccahon, R., Hardman, J. (2010). Pharmacology of plasma expanders. Anaesth Intens Care, 11:75–77.
- Burdick, W. (2004). A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med, 350:2247–2256.
- Gal, L. (1975). A review of the history of blood replacement from the 17th to the 20th centuries. Rev Surg, 32:229–239.
- Barron, M.E., Wilkes, M.M., Navickis, R.J. (2004). A systematic review of the comparative safety of colloids. Arch Surg, 139:552–563.
- Davidson, I.J. (2006). Renal impact of fluid management with colloids: a comparative review. Eur J Anaesthesiol, 23:721–738.
- Zekorn, D. (1969). Modified gelatin as plasma substitutes. Bibl. Haematol. 33:131–140.
- Ertmer, C., Rehberg, S., Aken, H., Westphal, M. (2009). Relevance of non-albumin colloids in intensive care medicine. Best Pract Res Clin Anaesthesiol, 23:193–212.
- Bateman, J.F., Harley, V., Chan, D., Cole, W.G. (1988). Comprehensive analysis of collagen metabolism in vitro using [43H]/[14C] proline dual-labeling and polyacrylamide gel electrophoresis. Anal Biochem, 168:171–175.
- Quasnichka, H.L., Tarlton, J.F., Anderson-Mackenzie, J.M., Billingham, M.E., Bailey, A.J., Pickford, A.R. (2005). Development of an assay for the quantification of type I collagen synthesis in the guinea pig. J Immunol Methods, 297:133–141.
- Walsh, B.J., Thornton, S.C., Penny, R., Breit, S.N. (1992). Microplate reader-based quantitation of collagens. Anal Biochem, 203:187–190.
- Deyl, Z., Novotná, J., Mikšík, I., Jelínková, D., Uhrová, M., Suchánek, M. (1997). Quantitation of collagen types I, III and V in tissue slices by capillary electrophoresis after cyanogen bromide solubilization. J Chromatogr B, 689:181–194.
- Yamaoka, T., Tabata Y., Ikada Y. (1994). Body distribution of intravenously administered gelatin with different molecular weights. J Control Release, 31:1–8.
- Bailey, A.J., and Paul, R.G. (1998). Collagen: a not so simple protein. J Soc Leach Tech Ch, 82:104–110.
- Schuller-Levis, G., Gordon, R.E., Wang, C.H., Park, S.Y., Parka, E. (2009). Protection of bleomycin-induced fibrosis and inflammation by taurine. Int Immunol, 9:971–977.
- Tsai, S.F., Liu, B.L., Liao, J.W., Wang, J.S., Hwang, J.S., Wang, S.C., Tzeng, Y.M., Ho, S.P. (2003). Pulmonary toxicity of thuringiensin administered intratracheally in Sprague–Dawley rats. Toxicology, 186:205–216.
- Aytekin, F.O., Teke, Z., Aydin, C., Kabay, B., Yenisey, C., Sacar, S., Demir, E.M., Tekin, K. (2007). Effects of a membrane-permeable radical scavenger, Tempol, on healing of colonic anastomoses in the cecal ligation and puncture model of polymicrobial sepsis in rats. Am J Surg, 193:723–729.
- Saddler, J.M., Horsey, P.J. (2007). The new generation gelatins: a review of their history, manufacture and properties. Anaesthesia, 42:998–1004.
- Tourtellotte, D., Williams, H.E. (1958). Acylated Gelatins and their Preparations. United States Patent, No. 2827419.
- Higgins, A.R., Harper, H.A., Kimmel, J.R., Burns, T.W., Jones, R.E., Smith, T.W.D., Klein, C.L. (1952). Oxypolygelatin - A plasma volume expander. Am J Med, 13:94.
- Nickerson, M., Patel, J., Heyd, D., Rousseau, D., Paulson, A. (2006). Kinetic and mechanistic considerations in the gelation of genipin-crosslinked gelatin. Int J Biol Macromol, 39:298–302.
- Koo, H.J., Song, Y.S., Kim, H.J., Lee, Y.H., Hong, S.M., Kim, S.J., Kim, B.C., Jin, C., Lim, C.J., Park, E.H. (2004). Antiinflammatory effects of genipin, an active principle of gardenia. Eur J Pharmacol, 495:201–208.
- Tseng, T.H., Chu, C.Y., Huang, J.M., Shiow, S.J., Wang, C.J. (1995). Protects against damage in rat primary hepatocytes. Cancer Lett, 97:61–67.
- Hou, Y.C., Tsai, S.Y., Lai, P.Y., Chen, Y.S., Chao, P.D.L. (2008). Metabolism and pharmacokinetics of genipin and geniposide in rats. Food Chem Toxicol, 46:2764–2769.
- Bigi, A., Cojazzi, G., Panzavolta, S., Roveri, N., Rubini, K. (2002). Stabilization of gelatin films by crosslinking with genipin. Biomaterials, 23:4827–4832.
- Yao, C.H., Liu, B.S., Chang, C.J., Hsu, S.H., Chen, Y.S. (2004). Preparation of networks of gelatin and genipin as degradable biomaterials. Mater Chem Phys, 83:204–208.
- Huang, K.S., Lu, K., Yeh, C.S., Chung, S.R., Lin, C.H., Yang, C.H., Dong, Y.S. (2009). Microfluidic controlling monodisperse microdroplet for 5-fluorouracil loaded genipin-gelatin microcapsules. J Control Release, 137:15–19.
- Nickerson, M.T., Patel, J., Heyd, D.V., Rousseau, D., Paulson, A.T. (2006). Kinetic and mechanistic considerations in the gelation of genipin-crosslinked gelatin. Int J Biol Macromol, 39:298–302.
- Liu, T., Zhang, G.F., Zhou, W.B., Su, Z.G. (2007). Determination of modification degree of succinylated gelatin by size exclusion chromatography coupled with multi angle laser light scattering. Chinese J Anal Chem, 35:43–48.
- Michael, M., Bernd, M. (2003). Characterization of gelatine and acid soluble collagen by size exclusion chromatography coupled with multi angle light scattering (SEC-MALS). Biomacromolecules, 4:1727–1732.
- Ye, H.P. (2006). Simultaneous determination of protein aggregation, degradation, and absolute molecular weight by size exclusion chromatography-multi angle laser light scattering. Anal Biochem, 356:76–85.
- Wyatt, P.J. (1997). Multiangle light scattering: the basic tool for macromolecular characterization. Instrum Sci Technol, 25: 1–18.
- Wyatt, P.J. (1993) Light scattering and the absolute characterization of macromolecules. Anal Chim Acta, 272:1–40.
- Bischoff, K.B., Dedrick, R.L. (1970). Generalized solution to linear, two-compartment, open model for drug distribution. J Theor Biol, 29:63–83.
- Shen, Z.J., Sun, Z.M., Wu, L., Wu, K., Sun, S.Q., Huang, Z.B. (2002). Rapid method for the determination of amino acids in serum by capillary electrophoresis. J Chromatogr A, 979:227–232.