Abstract
This experiment intended to authenticate the compensation and amplification effect of regenerated nerve fibers after nerve injury in primate. The Rhesus Monkeys right ulnar nerves and musculocutaneous nerves were chosed. The proximal impaired ulnar nerve as the proximal end and the distal impaired ulnar nerve musculocutaneous nerve as the distal ends. The ulnar nerve proximal stump fibers can grow into both the ulnar nerve distal stump and the musculocutaneus nerve at the same time and established two different electrophysiological conduction passageway. There exist nerve fibers compensation amplification effect after peripheral nerve injury on Rhesus Monkeys.
The functional recovery effect after peripheral nerve injury is dependent on the accurate connection of sensory and motor nerves, respectively [Citation1,Citation2]. In the past few years, bridging the peripheral nerve with a small gap for recovery of peripheral nerve injury has become a research focus gradually, and the microenvironment for neural regeneration supplied by biological conduits can fully profit from selectional regenerative actions of nerve fiber [Citation3,Citation4]. In our previous experiment on SD rats, we found that at the initial stage of neural regeneration, the proximal injured axons grew more than one new bud, and the number of proximal new-grown buds far exceeded the number of distal endoneurial tubes [Citation5–8]. On the foundation of this character, we hypothesized fewer nerve fibers can repair more nerve fibers at the distal end. To confirm this phenomenon, we designed a nerve fibers compensation amplification experiment repairing the distal ulnar nerve and musculocutaneous nerve with proximal ulnar nerve (A−A+B model) on Rhesus monkeys to authenticate the possibility that fewer proximal nerve fibers can dominate more distal nerve fibers.
MATERIALS AND METHODS
Materials: midheaven metuliform tube (a de-acetyl chitosan biological tube invented by Peking University People's Hospital, national patent number: 01136314.2). Dimension: length 10mm, thickness 1mm, inner diameter 4mm.
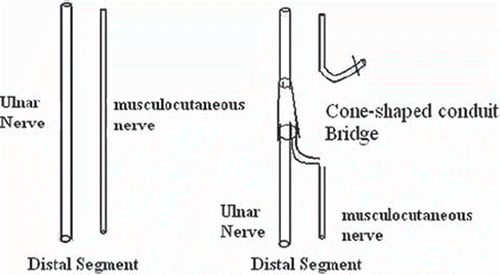
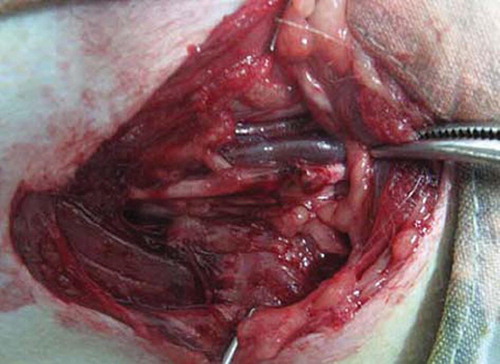
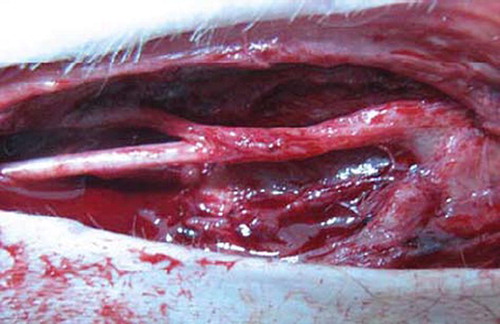
Groups: 8 male Rhesus monkeys (6.48 ± 1.24Kg) were anesthetized with SUMIANXIN ║(manufactured by Academy of Military Medical Sciences) via intramuscular injection at 0.11ml/kg. The left ulnar nerves at the elbow were exposed and disconnected at 6cm upper elbow humerus inner condylar. There was 2mm space between the distant and the near broken ends of nerve after sleeve joint ( and ). The right limbs were not handled to ensure the monkeys' life quality.
DETECTING ITEMS
At initial operation, we tested the motor nerve conduction velocity (MNCV) of ulnar nerves and musculocutaneous nerves exposing the left elbow ulnar nerve and regarded it as normal control value.
Observation in general: revealing the left ulnar nerve and musculocutaneous nerve of every experimental group with anaesthesia at months 3 and 6 post-operation, we observed the general shape of the conduit at suture and the adhesive condition of soft tissue.
Electrophysiologic examination: to have electrophysiologic examination, respectively, at months 3, 6 post-operation. A central stimulating electrode was put at the proximate neural conduit suture and the receiving electrodes at the muscle of the hypothenar and musculus biceps brachii, measuring interelectrode distance and difference of latent period, testing motor conduction velocity (MCV) with an Oxford electromyologram (EMG) meter at 3 months after operation. The MCV was also tested and some tissue was taken for histological analysis after 6 months.
STATISTICAL ANALYSIS
Data were expressed as mean±SD. The SPSS 11.0 software package was used for statistical analysis. Experimental data were compared using the t test, with p <0.05 considered statistical significance and comparing mean difference in nerve conduction velocity of each group.
RESULTS
Observation in General
Three months after operation, the conduit had been absorbed partly and remained its outline, and the blood vessel network was distinct on conduits. Six months after operation, the conduits outline were dim, but the proximal nerve trunk was smooth, almost the same to the distal segment ().
Motor Function Recovery of Diatal Limbs
Three months after operation the hand function innervated by the ulnar nerve recovered little and finger adduction and abduction did not appear, and the eating action was accomplished by holding using both hands. The elbow flexion action also did not appear. The finger adduction and abduction action were observed after 6 months and the elbow flexion action ranged 20-40 degrees.
Electrophysiological Testing at 3 Months after Operation
Putting the stimulating electrode at proximal ulnar nerves, the action potential was recorded at distal biceps brachii and hypothenar muscles, respectively. The NCV (nerve conduction velocity) is 14.9267 ± 1.0983m/s from the proximal ulnar nerves to musculocutaneous nerves, far lower than 44.5259 ± 2.1981m/s to control value. The NCV is 20.7950 ± 2.1857mm/s from the proximal ulnar nerve to distal ulnar nerve, also lower than 49.2328 ± 2.1293m/s as the normal control.
Electrophysiological Testing 6 Months after Operation
Putting the stimulating electrode at the proximal ulnar nerves, the action potential was recorded at distal biceps brachii and hypothenar muscles, respectively. The NCV (nerve conduction velocity) is 16.2467 ± 0.8262m/s from the proximal ulnar nerves to musculocutaneous nerves, far lower than the 44.5259 ± 2.1981m/s control value. The NCV is 23.8867 ± 0.7876m/s from the proximal ulnar nerve to distal ulnar nerve, also lower than the 49.2328 ± 2.1293m/s control value.
The ratio of (the myelinated fiber numbers of distal ulnar nerves + the myelinated fiber numbers of distal musculocutaneous nerves)/(the myelinated fiber numbers of proximal ulnar nerves) was 1.2884 ± 0.0487 in histological osmium tetroxide staining.
DISCUSSION
Peripheral nerve injury is very common in clinics. Peripheral nerve selective regeneration based on “Y-tube experiments” provided a new, practical way for good docking of nerve fibers during regeneration. Jiang Baoguo [Citation9,Citation10] et al., developed a degradable conduit and confirmed that conduit sleeve bridging small gap (2mm) could promote the restoration of SD rat sciatic nerve injury. This new repair method had the possibility for substituting the traditional used epineurial neurorrhaphy or perineurium neurorrhaphy for nerve injury [Citation11,Citation12]. Regeneration room formed by the biological conduit and nerve stumps may benefit the injured nerve axon regeneration.
During this process to confirm the phenomenon we also found: at the beginning regeneration phase, proximal injured nerve fiber grew several lateral buds; some of these buds grew into the distal correspondingmyelin sheath tube and some of them became gradually atrophied. In the SD rat sciatic nerve injury small gap conduit sleeve bridge model, even after 3-6 months, the number of myelinated nerve fibers in the distal segment was much larger than that of the proximal segment. Considering that the quality of the distal myelinated nerve fiber was quite good, we supposed that a small number of nerve fibers can innervate a relatively larger number nerve fibers. We named it “nerve fibers compensation amplification innervations.” If this phenomenon is true, it may provide us with a new method to repair nerve injury by making good use of this nerve fibers compensation amplification innervation effect [Citation13].
Therefore we designed this experiment to confirm this nerve fibers compensation amplification innervation effect in primate animal model. In this model, we used the ulnar nerve proximal stump to repair both the ulnar nerve distal stump and the musculocutaneus nerve at the same time. Namely, we tried this A−A+B repair model. In fact, the ulnar nerve about the elbow of rhesus monkesys was composed of sensory nerves and motor nerve fibers while the musculocutaneus nerve here was composed of almost all motor nerve fibers.
If this phenomenon or theory proved to be true, the nerve fibers of the proximal ulnar nerve will grow into a distal ulnar nerve and musculocutaneus nerve, respectively, and form two different electrophysiological conduction passageways, and may recover partial function of distal target organs. During the detection process, we stimulated the ulnar nerve proximal segment, and recorded action potential both in the musculus biceps brachii innervated by the musculocutaneus nerve and in the distal hypothenar muscle innervated by the ulnar nerve. It proved to be true in this experiment that the ulnar nerve proximal stump fibers can grow into both the ulnar nerve distal stump and the musculocutaneus nerve at the same time and establish two different electrophysiological conduction passageways.
In this experiment model (A−A+B repairing model), the motor nerve conduction velocity from the proximal segment of the ulnar nerve to the distal segment of the ulnar nerve was about 42 % of the ulnar nerve normal control after 3 months, and about 48 % of the ulnar nerve normal control after 6 months. The motor nerve conduction velocity from the proximal segment of the ulnar nerve to the distal segment of the musculocutaneus nerve was about 33 % of the ulnar nerve normal control after 3 months, and about 37 % of the ulnar nerve normal control after 6 months. In our previous research about the Rhesus monkey median nerve injury biological conduit small gap bridge model, we also found: The motor nerve conduction velocity recovery was apparently higher than that of the conventional perilemma suture group, but just equal to 56%-60% of normal control value, even after 6 months. In our experiment, we also calculated the ratio of (the myelinated fiber numbers of distal ulnar nerves + the myelinated fiber numbers of distal musculocutaneous nerves)/(the myelinated fiber numbers of proximal ulnar nerves) was 1.2884 ± 0.0487 in histological osmium tetroxide staining. We also found matured myelinated fiber in the distal musculocutaneus nerve, and this may challenge us with this phenomenon: matured ulnar nerve can innervate the injured musculocutaneus nerve, although the quality and character of these two different nerves were quite different.
This experiment may not be enough to explain the exact mechanism of this phenomenon, so it needs to be further explored in this aspect: The histological observation had displayed that the ratio of (the myelinated fiber numbers of distal ulnar nerves + the myelinated fiber numbers of distal musculocutaneous nerves)/(the myelinated fiber numbers of proximal ulnar nerves) was 1.2884 ± 0.0487; the magnification and compensation effect of the proximal ulnar nerve segment may exist. But considering the fiber number and quality difference between the ulnar nerve and musculocutaneus nerve, this model may not be the most suitable model to explore the magnification and compensation effect. Because the musculocutaneus nerve fiber number was only about 1/6-1/8 of the ulnar nerve, this difference may not permit the magnification and compensation to produce a marked effect fully in this model. But we summarized that the magnification and compensation effect in this model exist and injured proximal ulnar nerve can innervate the injured distal segment of the musculocutaneus nerve. This phenomenon or regeneration theory may provide us with a new nerve repair method for special nerve injury with the magnification and compensation effect.
Table 1. Motor nerve conduction velocity testing after 3 months
Table 2. Motor nerve conduction velocity testing after 6 months
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Lundborg, G. (2000). A 25-year perspective of peripheral nerve surgery: evolving neuroscientific concepts and clinical significance. J Hand Surg Am. 25(3):391–414.
- Jian, Li, Baoguo, Jiang, Dianying, Zhang, . (2003). Biological tube small gap bridging repair peripheral nerve injury. Chinese Hand Surgery Journal, 19(2):118–120.
- Wang, Y.H., Zhang, D.Y., Zhang, P.X., (2009). Amplification effects in nerve regeneration after different segment injury: experiment with rabbit median nerve. Zhonghua Yi Xue Za Zhi. 89(23):1645–9.
- Zhao, F.Q., Zhang, P.X., Jiang, B.G. (2007). Magnifying effect of conduit bridging in number of nerve fibers of broken peripheral nerves: experiment with rats. Zhonghua Yi Xue Za Zhi. 17:87(15):1043–7.
- Jiang, B.G., Yin, X.F., Zhang, D.Y., . (2007). Maximum number of collaterals developed by one axon during peripheral nerve regeneration and the influence of that number on reinnervation effects. Eur Neurol. 58(1):12–20.
- Jiang, B., Zhang, P., Jiang, B. (2010). Advances in small gap sleeve bridging peripheral nerve injury. Artif Cells Blood Substit Immobil Biotechnol. 38(1):1–4.
- Yu, K., Zhang, C., Wang, Y., (2009). The protective effects of small gap sleeve in bridging peripheral nerve mutilation. Artif Cells Blood Substit Immobil Biotechnol. 37(6):257–64.
- Zhang, P., Zhang, C., Kou, Y., . (2009). The histological analysis of biological conduit sleeve bridging rhesus monkey median nerve injury with small gap. Artif Cells Blood Substit Immobil Biotechnol. 37(2):101–4.
- Zhang, P., Yin, X., Kou, Y., (2008). The electrophysiology analysis of biological conduit sleeve bridging rhesus monkey median nerve injury with small gap. Artif Cells Blood Substit Immobil Biotechnol. 36(5):457–63.
- Zhang, P., Xue, F., Kou, Y., . (2008). The experimental study of absorbable chitin conduit for bridging peripheral nerve defect with nerve fasciculu in rats. Artif Cells Blood Substit Immobil Biotechnol. 36(4):360–71.
- Zhang, P., Xue, F., Zhao, F., (2008). The immunohistological observation of proliferation rule of Schwann cell after sciatic nerve injury in rats. Artif Cells Blood Substit Immobil Biotechnol. 36(2):150–5.
- Jiang, B., Zhang, P., Zhang, D., (2006). Study on small gap sleeve bridging peripheral nerve injury. Artif Cells Blood Substit Immobil Biotechnol. 34(1):55–74.
- Zhang, P., He, X., Zhao, F., (2005). Bridging small-gap peripheral nerve defects using biodegradable chitin conduits with cultured schwann and bone marrow stromal cells in rats. J Reconstr Microsurg. 21(8):565–71.