Abstract
This study was designed to compare the influence of deoxygenated hemoglobin-based oxygen carrier (HBOC) pretreatment and ischemia preconditioning on cardiac ischemia/reperfusion (I/R) injury. Langendorff-perfused rat hearts were pretreated with 0.1 gHb/dL deoxygenated HBOC or ischemia, then subjected to 50-min warm ischemia and 2-hr reperfusion. The results indicated that deoxygenated HBOC pretreatment and ischemia preconditioning both equally improved the recovery of cardiac function, and reduced the cardiac enzyme release and myocardial histopathological changes as compared to the control group. Therefore our study demonstrated that deoxygenated HBOC pretreatment and ischemia precondition provided equivalent protection to the isolated heart against I/R injury.
INTRODUCTION
Ischemia/reperfusion (I/R) injury is harmful to the cardiovascular system and responsible for cardiac infarction and ischemic heart disease [Citation1–3]. Reduction of cardiac I/R injury improves the survival and prognosis of patients, which is urgently required in clinic settings. As we know, ischemia preconditioning is commonly recognized as a promising method of cardioprotection [Citation4–6]. In other words, repetitive brief episodes of I/R are beneficial to decreasing the following I/R injury.
Our previous studies have demonstrated that oxygen-saturated polymerized human placenta hemoglobin (PolyPHb), one type of hemoglobin-based oxygen carrier (HBOC), can protect the isolated rat heart from I/R injury, and the underlying mechanisms are implicated in attenuation of myocardial apoptosis, quenching cardiac mitochondrial oxidative stress and restoration of nitroso-redox balance [Citation7–10]. However, the influence of deoxygenated HBOC on the I/R heart has never been reported, and whether deoxygenated HBOC pretreatment provides the same cardioprotection as ischemia preconditioning is poorly understood. Therefore we designed this study to investigate the influence of deoxygenated HBOC pretreatment on the isolated rat heart after warm ischemia and reperfusion, and compared with the effect of ischemia preconditioning.
MATERIALS AND METHODS
The present study was performed in adherence with the Guidelines on the Use of Laboratory Animals published by the National Institutes of Health and approved by the Animal Care and Use Committees in Sichuan University.
HBOC Solution Preparation
HBOC in this study was PolyPHb, which was prepared as we previously described [Citation11,Citation12]. Before use, the HBOC solution was added into a Krebs-Henseleit buffer (KHB: 120.0 mM NaCl, 4.5 mM KCl, 20.0 mM NaHCO3, 1.2 mM KH2PO4, 1.2 mM MgCl2, 2.5 mM CaCl2 and 10.0 mM glucose, pH 7.4, 37°C) to a final concentration of 0.1 gHb/dL, and bubbled with 95% nitrogen gas + 5% carbon dioxide for 10 mins for deoxygenation. As a result, the oxygen tension of the above HBOC solution was 42+15 mmHg, which was significantly lower than that of the KHB alone (532±25 mmHg).
Experimental Proposal
Forty male Sprague-Dawley rats, weighing 250–300 g, were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg) and heparin (500 IU). The hearts were quickly excised and perfused on a Langendorff apparatus with KHB at a constant pressure of 100 cmH2O. A thin-wall latex balloon was inserted into the left ventricle (LV) through the left atrium to continuously monitor the LV function, including left ventricular developed pressure (LVDP), maximum LVDP increase (+dp/dt) and decrease rate (−dp/dt), and LV end-diastolic pressure (LVEDP) (AD Instruments Pty Ltd., Bella Vista, NSW, Australia). Coronary flow rate (CF) was calculated from the coronary effluent, sampling time, and cardiac wet weight.
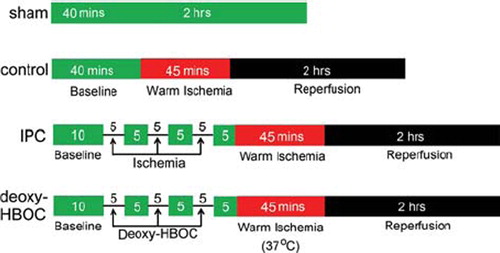
As shown in , after 10 mins of basal perfusion, hearts were randomly assigned to sham, control (without pretreatment), IPC (ischemia preconditioning), and deoxy-HBOC groups (pretreatment with deoxygenated HBOC). In the IPC and deoxy-HBOC groups, a sequence of three 5-min deoxygenated HBOC perfusion or ischemia and 5-min KHB perfusion was performed, respectively. The control group hearts were basal perfused for 40 mins without pretreatment. Then the hearts were subjected to 50-min ischemia and 2-hr reperfusion. The sham group hearts were perfused by KHB for 160 mins without pretreatment and I/R injury.
Figure 1. The experimental protocol of this study. In the IPC and deoxy-HBOC groups hearts, a sequence of three 5-min deoxygenated HBOC perfusion or ischemia and 5-min KHB perfusion were performed, then subjected to 45 mins of ischemia at 37°C and 2 hrs reperfusion. Hearts suffered from I/R injury without pretreatment were used as the control group. Hearts perfused with KHB for 160 mins without pretreatment and I/R injury were used as the sham group. KHB: Krebs-Henseleit buffer.
Cardiac Enzyme Release Measurement
The coronary effluents at the end of 10-min baseline and 2-hr reperfusion were collected; the release of cardiac enzyme, including creatine kinase-MB (CK-MB) and lactate dehydrogenase (LDH), was measured by an Olympus AU5400 autoanalyser (Olympus Diagnostics, Melville, NY) within 2 hrs.
Myocardial Histopathological Analysis
After 2 hrs of reperfusion, the LV tissues were immediately fixed for 24 hours in 4% paraformaldehyde in 0.1 M phosphate buffer saline (pH 7.4), and then dehydrated in a series of ethanol and embedded in paraffin. After that, 4-μm sections were prepared and stained with hematoxylin and eosin (HE). The result of HE staining was assessed in a blinded fashion by a pathologist for the following histological examination: acute myocardial necrosis, cellular swelling, and fatty changes.
Statistical Analysis
All values in the text and figures were presented as mean ± SEM. The values of LVDP, ±dp/dt, LVEDP and CF were analyzed by 2-factor ANOVA with repeated measures. The releases of CK-MB and LDH were analyzed by one-way ANOVA followed by LSD correction for post hoc t test (SPSS 13.0 software). P values < 0.05 were considered statistically significant.
RESULTS
Deoxygenated HBOC Pretreatment Increased LV Function
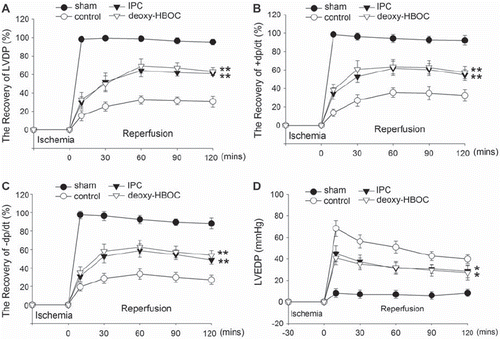
There were no significant differences in LVDP, ±dp/dt, LVEDP and CF among the 4 groups at basal perfusion, but during reperfusion the LVDP was greatly improved in the IPC and deoxy-HBOC groups as compared with the control group (P < 0.01 and P < 0.01, respectively, ). Both the +dp/dt and −dp/dt were also greatly increased in the IPC and deoxy-HBOC groups when compared to the control group (P < 0.01 and P < 0.01, respectively, and ). In addition, the LVEDP of the control group was greatly elevated during reperfusion; however, ischemia preconditioning and deoxygenated PolyPHb pretreatment significantly reduced its elevation (P < 0.05 and P < 0.05, respectively, ). There was no significant difference in the CF among the 4 groups during reperfusion (data not shown). When we compared the cardiac function of the IPC group and deoxy-HBOC group, we found there were no significant differences.
Figure 2. The cardiac function recovery of LVDP (A), ±dp/dt (B and C) and LVEDP (D) of the 4 group hearts. Values were presented as mean ± SEM (n=10). *P < 0.05 and **P < 0.01 vs. the control group. LVDP: left ventricular development pressure, ±dp/dt: maximum LVDP increase and decrease rate, LVEDP: left ventricular end-diastolic pressure.
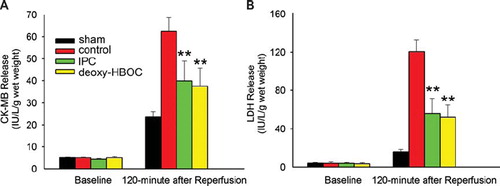
Deoxygenated HBOC Pretreatment Reduced CK-MB and LDH Release
At baseline, the levels of CK-MB and LDH release were low and showed no significant difference among the 4 groups. After 2-hr reperfusion, both of them were up-regulated in the control group (62.34 ± 6.33 IU/L/g wet heart for CK-MB release and 120.51 ± 12.37 IU/L/g wet heart for LDH release). Ischemia preconditioning and deoxygenated HBOC pretreatment exhibited equally inhibitory effects on the reduction of CK-MB and LDH release (CK-MB release: P < 0.01 and P < 0.01 vs. the control group, respectively; LDH release: P < 0.01 and P < 0.01 vs. the control group, respectively, ).
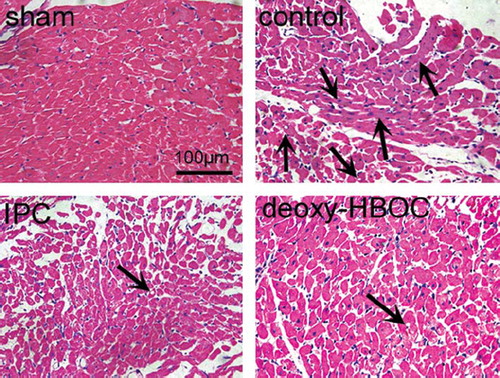
Deoxygenated HBOC Pretreatment Limited Myocardial Histopathological Changes
As shown in , after I/R injury, the extents of acute myocardial necrosis, cellular swelling and fatty changes (arrows) were greatly augmented in the control group as compared to the sham group. However, these histopathological changes were largely lessened in the IPC and deoxy-HBOC groups.
DISCUSSION
HBOC was initially developed as an oxygen-carrying agent for emergency use when traditional blood transfusion is unavailable or unsafe [Citation13–16]. Besides, more and more studies have demonstrated its cardioprotective effect against I/R injury, which revealed an alternative clinical use of HBOC [Citation7–10,Citation17,Citation18]. As a promising oxygen carrier, HBOC is able to freely diffuse in microcirculation and transport oxygen to hypoxia tissues owing to its high oxygen affinity, low viscosity and small mean diameter. However, the effect of deoxygenated HBOC on cardiac I/R injury has never been investigated. In the present study, we compared the influence of deoxygenated HBOC pretreatment and ischemia preconditioning on the I/R heart, and the results demonstrated that there were no significant differences of cardiac functional recovery, enzyme release, and histopathological changes between these two groups. Both deoxygenated HBOC pretreatment and ischemia preconditioning greatly improved the cardiac contractile performance recovery, as evidenced by the elevated LVEDP and ±dp/dt and reduced LVEDP during reperfusion. In addition, the CK-MB and LDH release and histopathological changes were also equally inhibited in the IPC and deoxy-HBOC groups.
In my opinion, just like ischemia preconditioning, the cardioprotective effect of deoxygenated HBOC pretreatment can also be attributed to repetitive brief episodes of I/R, because deoxygenated HBOC pretreatment induced a sequence of hypoxia and reperfusion to myocardium too. As we know, deoxygenated HBOC can take away the oxygen preloaded to myocardium during basal perfusion instead of providing oxygen. Therefore, in theory, deoxygenated HBOC would induce a higher level of hypoxia as compared to ischemia, thus deoxygenated HBOC pretreatment might provide more protection to the I/R heart than ischemia preconditioning. Unfortunately, our results found that there was no significant difference between these 2 groups. Another important finding of this study is that hypoxia may be the key factor mediated the cardioprotection of ischemia preconditioning and hypoxia preconditioning, because hypoxia preconditioning produced by deoxygenated HBOC pretreatment had similar cardioprotective effect as ischemia preconditioning in the present study. We believe the method of deoxygenated HBOC pretreatment can be widely used in clinical situations; for example, it can be performed on the patient before cardiopulmonary bypass to protect the myocardium.
There were some limitations of this study. First, in the clinic situation, ischemia preconditioning is not an ideal method of cadioprotection; we cannot block the aorta to induce cardiac ischemia. However, according to regulation of the fraction of inspired oxygen, hypoxia preconditioning is easy to perform. We probably should employ hypoxia preconditioning as a control instead of ischemia preconditioning. Second, even though we demonstrate that deoxygenated HBOC pretreatment and ischemia preconditioning had similar cardioprotective effects by the results of cardiac function recovery, enzyme release, and histopathological changes. However, these results were far from enough to support that deoxygenated HBOC pretreatment and ischemia preconditioning shared similar mechanisms of cardioprotection.
In conclusion, the present study provided distinct evidence that deoxygenated HBOC pretreatment provided protection to the isolated heart against I/R injury and this protection was equivalent to the ischemia preconditioning, which revealed the alternative clinic uses of HBOC in cardiac-related surgery.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Becker, L.B. (2004). Cardiovasc Res. 61:461–470.
- Bolli, R., Becker, L., Gross, G., Mentzer, R., Jr., Balshaw, D., Lathrop, D.A. (2004). Circ Res. 95:125–134.
- Shibata, R., Sato, K., Pimentel, D.R., Takemura, Y., Kihara, S., Ohashi, K., Funahashi, T., Ouchi, N., Walsh, K. (2005). Nature Medicine. 11:1096–1103.
- Hoole, S.P., Heck, P.M., Sharples, L., Khan, S.N., Duehmke, R., Densem, C.G., Clarke, S.C., Shapiro, L.M., Schofield, P.M., O'Sullivan, M., Dutka, D.P. (2009). Circulation. 119:820–827.
- Churchill, E.N., Ferreira, J.C., Brum, P.C., Szweda, L.I., Mochly-Rosen, D. (2010). Cardiovascular Research. 85:385–394.
- Hausenloy, D., Wynne, A., Yellon, D. (2007). Basic Research in Cardiology. 102:445–452.
- Li, T., Li, J., Liu, J., Zhang, P., Wu, W., Zhou, R., Li, G., Zhang, W., Yi, M., Huang, H. (2009). Free Radical Biology and Medicine. 46:397–405.
- Li, T., Zhang, P., Liu, J., Zhou, R., Li, Q., You, Z., Dian, K. (2010). Free Radical Biology and Medicine. 48:1079–1089.
- Li, T., Liu, J., Yang, Q., Wu, W., Zhang, P., Yang, J., Li, J., Zhang, W., Yang, C. (2009). Artif Cells Blood Substit and Biotechnol. 37:48–52.
- Li, T., Liu, J., Yang, C. (2010). Artif Cells Blood Substit and Biotechnol. DOI: 10.3109/10731191003670541.
- Li, T., Yu, R., Zhang, H.H., Wang, H., Liang, W.G., Yang, X.M., Yang, C.M. (2006). Artif Cells Blood Substit and Biotechnol. 34:175–188.
- Li, T., Zhang, H., Wang, H., Yu, R., Yang, C. (2006). Journal of Biomedical Engineering. 23:640–644.
- D'Agnillo, F., Chang, T.M. (1998). Nat Biotechnol. 16:667–671.
- Stowell, C.P. (2002). Current Opinion in Hematology. 9:537–543.
- Alayash, A.I. (1999). Nat Biotech. 17:545–549.
- Silverman, T.A., Weiskopf, R.B., for the Planning, C.; The, S. (2009). Anesthesiology. 111:946–963.
- George, I., Yi, G.H., Schulman, A.R., Morrow, B.T., Cheng, Y., Gu, A., Zhang, G., Oz, M.C., Burkhoff, D., Wang, J. (2006). Am J Physiol Heart Circ Physiol. 291:1126–1137.
- Burmeister, M.A., Rempf, C., Standl, T.G., Rehberg, S., Bartsch-Zwemke, S., Krause, T., Tuszynski, S., Gottschalk, A., Schulte am Esch, J. (2005). Br J Anaesth. 95:737–745.