Abstract
The propensity of site-specific carboxymethylation and Propylation of Val-1(α) of Hb to attenuate the reductive hexaPEGylation-induced dissociation of tetramers has been investigated. Only reductive Propylation of Val-1(α), which increases the stability of oxy Hb, attenuates the reductive hexaPEGylation-induced dissociation. Increasing the stability of the oxy conformation of Hb by chemical or genetic approaches is a strategy to generate PEGylated Hbs with native-like tetramer stability using direct PEGylation platforms. This new approach and EAF-PEGylation are the only two alternate PEGylation strategies available to design stable second-generation vasoinactive uncrosslinked PEGylated Hbs with native-like tetramer stability.
INTRODUCTION
PEGylation induced plasma expander-like properties of Hb are considered as the molecular aspects of PEGylated Hbs that make them vasoinactive and nonhypertensive [Citation1–3]. Extension arm facilitated (EAF) PEGylation has been developed to take advantage of this aspect of PEGylation and simplify the PEGylation platform. EAF PEGylation has turned out to be a very simple and cost-effective platform to generate a vasoinactive hexaPEGylated Hb, (SP-PEG5K)6-Hb [Citation4,Citation5]. MP4 is a EAF hexaPEGylated Hb (Sangart Inc., CA) that has already gone through Phase III clinical trial as an oxygen-carrying plasma expander and is a prototype of (SP-PEG5K)6-Hb [Citation6,Citation7].
Sangart's choice of this PEGylation pattern is, apparently, related to its high oxygen affinity as it facilitates the targeting of the oxygen to oxygen-starved organs and tissues, the simplicity of the EAF PEGylation platform, the homogeneity of the molecular size of the PEG-Hb adducts, and it is generated from uncrosslinked Hb [Citation6]. This high oxygen affinity of the PEGylated Hb reduces or eliminates autoregulation-mediated vasoconstriction. The high oxygen affinity of (SP-PEG5K)6-Hb has been attributed to the direct PEGylation (without the extension arm) of Cys-93(β) [Citation8]. However, subsequent studies have shown that when Cys-93(β) is protected and the EAF-PEGylation is targeted exclusively to the amino groups, the PEGylated Hb is still a high oxygen affinity species. Thus increase in the oxygen affinity of Hb appears to be a function of PEG-shell and not a direct correlate of PEGylation of Cys-93(β) [Citation9].
Reductive alkylation using PEG5K propionaldehyde was used as an alternate PEGylation platform for hexaPEGylation of Hb without modifying Cys-93(β) [Citation10]. (Propyl-PEG5K)6-Hb exhibited a higher oxygen affinity than (SP-PEG5K)6-Hb and the product exists essentially as PEGylated dimers of Hb under the physiological conditions [Citation11]. Subsequent studies have shown that hexaPEGylated Hbs generated by acylation chemistry-mediated PEGylation as well as thiocarbamoylation chemistry-mediated PEGylation are also high oxygen affinity species and also exist as PEGylated dimers [Citation12,Citation13].
On the contrary, (SP-PEG5K)6-Hb, PEGylated Hb generated by EAF-PEGylation, exists predominantly as tetramers under the physiological conditions. This is a unique and distinguishing feature of EAF-PEG-Hbs vs other PEG-Hbs; for example, the PEG-bovine Hb of Enzon. EAF-PEGylation targeted exclusively to the ε-amino groups through reversible protection of Cys-93(β) during EAF-PEGylation, the hexaPEGylated product exhibits the tetramer stability comparable to that of an unmodified tetramer [Citation9]. We have now established that all direct PEGylation protocols that modified the Hb directly without the introduction of the extension arms (acylation chemistry, thiocarbamoylation chemistry, reductive alkylation chemistry, and succinimidyl carbonate chemistry based PEGylation) generate hexaPEGylated Hbs that exist predominantly as PEGylated dimmers of Hb. Intramolecularly crosslinking of Hbs have been used as the strategy by us to overcome the PEGylation promoted dissociation of the tetramer into dimmers, particularly to generate PEG Hbs with lower oxygen affinity [Citation11]. These results suggest that PEG chains on the molecular surface of Hb exert a strong influence within the central cavity and that the extension arms engineered between the PEG-chains and the protein amino groups attenuate the influence of PEGylation on the interdimeric interactions of Hb and the only approach available to avoid any tetramer dissociation mediated toxicities in vivo. Consistent with this concept of attenuation of the PEGylation-based dissociation of the tetramer by the extension arms, promotion of the tetramer dissociation facilitated by the acylation PEGylation is attenuated by introduction of extension arms (aliphatic chains) between the PEG chains and protein amino groups, still using acylation chemistry to introduce the extension arm. Accordingly, we have advanced the concept that developing extension arm chemistry versions of all direct PEGylation platforms using the respective conjugation chemistries to introduce extension arms and with thiol or maleimide moieties at the distal end of the extension arms and using maleimide PEG or thioPEG as the PEG-reagent as a general strategy to attenuate the PEGylation promoted dissociation of Hb tetramers [Citation15].
The promotion of the tetramer dissociation by direct PEGylation could be a site-specific PEGylation effect, or a generalized effect of PEGylation at multiple sites on the protein hydration layer leading to the perturbation of the structure of bound water within the central cavity [Citation14]. Recently, we have established that attachment of Propyl PEG-5K chains on Val-1(α) and Val-1(β) increase the dissociation constant (Kd) of Hb by nearly 300- and 10-fold, respectively [Citation11], and this supports the concept that PEGylation promoted dissociation of the tetramer as a site-specific effect. This implies that if we should avoid the PEGylation at these sites by pre-modifying the site by a site-specific chemical modification reaction, the direct PEGylation promoted attenuation of Hb could be attenuated.
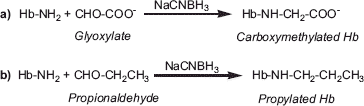
Accordingly, it is conceivable that the reductive PEGylation-induced dissociation of Hb tetramer will be attenuated by avoiding the PEGylation at the amino terminus of Hb by pre-modifying the Hb with small molecular weight reagents targeted to the α-amino groups and we have undertaken that study here. Reductive carboxymethylation of Hb on Val-1(α) or Val-1(β) by carboxymethylation using glyoxylic acid () has been chosen as the chemical strategy to avoid the reductive PEGylation at the respective sites. The carboxymethylation at these sites by itself has very limited influence on the interdimeric interactions of the tetramer. Besides, reductive population of Val-1(α) of Hb () has also been carried out as a new substrate for PEGylation, in view of the fact that the propylation of Hb at this site increases its inter dimeric interactions of the tetramer by nearly an order of magnitude.
Figure 1. Schematic representation of carboxymethylation (a) and propylation (b) of Hb through reductive alkylation chemistry.
The results presented here establish that avoiding the PEGylation at the α-amino groups of Hb by carboxymethylation does not increase the stability of the reductively hexaPEGylated carboxymethylated-Hbs. On the other hand, avoiding the PEGylation of Hb at Val-1(α) during reductive hexaPEGylation of Hb at this site by reductive propylation attenuates the reductive PEGylation-promoted dissociation of the tetramer. The Val-1(α) propylation-mediated stabilization of inter tetrameric interactions of Hb apparently contributes significantly to the stability of hexaPEGylated [Val-1(α) Propyl-]-Hb. The hexaPEGylated [Propyl-Val-1(α)] Hb exhibits an apparent dissociation constant comparable to that of unmodified Hb. In fact, this hexaPEGylated Hb appears to be even more stable than (SP-PEG5K)6-Hb. We speculate that Hb and/or modified Hbs stabilized at Val-1(α) by the attachment of alkyl chains (propyl or higher homologues) will result in the stabilization of reductively PEGylated Hbs. Advantages of this new hexaPEGylation platform that generates PEG-Hbs with native-like tetramer stability in the design of novel HBOC are discussed and compared with that of EAF-PEGylation.
MATERIALS AND METHODS
Preparation of [Propyl-Val-1(α)]2-Hb
Human adult hemoglobin (HbA) was purified from human erythrocytes as described previously [Citation16]. IHP-bound HbA (0.25 mM) was reacted with 1.5 mM propionaldehyde (Sigma, MO) and 7.5 mM NaCNBH3 (Sigma Chemical Co., MO) in 50 mM BisTris-Ac buffer (pH 6.5) overnight at 4°C. The reaction mixture was dialyzed against 50 mM Tris-Ac buffer (pH 6.0) and loaded on a Mono S column (0.5 × 5 cm2). The column was equilibrated by 50 mM Tris-Ac buffer (pH 6.0) and eluted by 40 column volume of 50 mM Tris-Ac buffer using a pH gradient of 6.0–7.0 at a flow rate of 0.8 ml/min. The desired fractions were pooled, concentrated and dialyzed against PBS buffer (pH 7.4).
Reductive Carboxymethylation of Hb
HbA was carboxymethylated using glyoxylate as described previously [Citation17]. Briefly, 0.5 mM HbA was incubated with 2 mM glyoxylate and 25 mM sodium cyanoborohydride at 4°C in 50 mM Bis-Tris buffer, pH 6.5 for 4 h. The excess unreacted materials were removed by dialyzing against PBS at 4°C overnight with 13,000 Mw cut off dialyzing membrane. The product was further dialyzed against Tris-acetate buffer, pH 8.0 at 4°C for 4 h and then applied to a Q-Sepherose high performance column (2.6 × 65 cm, 350 mL of column volume, Pharmacia). The column was equilibrated with 50 mM Tris acetate at pH 8.0 (buffer A) using an AKTA Purifier 10 System (Amersham Pharmacia Biotech.), and eluted at 4°C with two linear gradient of 0–90% buffer B (50 mM Tris acetate at pH 6.8) in 6 column volumes and then 90–100% buffer B in 8 column volumes. The column was eluted at a flow rate of 2 ml/min. The effluent was monitored at 540 and 630 nm. The peaks were collected and dialyzed against PBS overnight, and then concentrated and stored in −80°C freezer.
HexaPEGylation of Hb Derivatives
[Propyl-Val-1(α)]2-Hb, [Cm-Val-1(α)]2-Hb, or [Cm-Val-1(β)]2-Hb (0.25 mM) was reacted with 10 mM PEG5K propionaldehyde (Sunbio Inc., Korea) and 50 mM NaCNBH3 in 50 mM Bis-Tris-Ac buffer (pH 6.5) at 4°C overnight. The resultant products were dialyzed extensively against PBS buffer (pH 7.4). For removal of free PEG, the sample was centrifuged at 6,000 g using Centricon with 50 K cut-off membrane for 5 times. The retentate was concentrated and stored at −80°C.
Reverse Phase HPLC Analysis
Reverse phase HPLC analysis of the globin chains was carried out on a Vydac C4 column (0.46 × 25 cm2). The column was eluted with a linear gradient of 35–50% acetonitrile containing 0.1% TFA in 100 min, followed by a linear gradient of 50–70% acetonitrile containing 0.1% TFA in 30 min at a constant flow rate of 1.0 ml/min.
Tetramer-dimer Dissociation Constant
The tetramer-dimer dissociation constant (Kd) of the PEGylated Hbs was measured essentially as described by Manning et al. [Citation18]. Briefly, Hb samples in PBS buffer (pH 7.4) were diluted to a series of Hb concentrations and subjected to two Superose 12 columns (1 × 31 cm2) in series. The columns were equilibrated and eluted with PBS buffer (pH 7.4) at a flow rate of 0.5 ml/min. αα-fumaryl Hb was set as the tetramers for HbA and [Propyl-Val-1(α)]2-Hb. HexaPEGylated αα-Hb has been taken as the standard tetramer for the hexaPEGylated propyl-Hb.
Oxygen affinity measurements
Oxygen equilibrium curves of the Hb samples (0.5 mM) were measured using Hem-O-Scan (Aminco) at 37°C in PBS buffer (pH 7.4).
RESULTS
Dimer Tetramer Equilibrium of Site-selectively Modified Hbs
presents the dissociation constants of some site-selectively modified Hbs. Carboxymethylation of Hb at the amino terminus of α and/or β-chains, as well as NEM modification of Cys-93(β), induce very limited influence on the inter dimeric interactions of Hb. On the other hand, Hb site-selective propylation at Val-1(α) lowers the dissociation constant of Hb by nearly an order of magnitude, i.e strengthens the interdimeric interactions of the tetramer.
Table 1. Tetramer-Dimer Dissociation Constants of Modifed Hbs.
The Hb derivatives site-selectively modified at the amino terminus have been used for reductive hexaPEGylation to avoid the PEGylation at the respective sites. During reductive hexaPEGylation of Hb, Val-1(α) and Val-1(β) are PEGylated quantitatively, and by using these carboxymethylated and propylated Hbs, PEGylation at these sites are avoided, and accordingly can generate a PEG-Hb adduct with a native-like tetramer stability, if the PEGylation at the respective sites is directly responsible for the enhanced tetramer dissociation.
Avoiding the PEGylation of Either Val-1(α) or Val-1(β) does not Generate HexaPEGylated Hb with Native-like Tetramer Stability
The elution position of the two carboxymethylated Hb is comparable to that of HbA, demonstrating that carboxymethylation by itself does not influence the apparent hydrodynamic volume of Hb. As shown in , reductive hexaPEGylation of [Cm-Val-1(α)]2-Hb and [Cm-Val-1(β)]2-Hb generates products that exhibit an FPLC elution pattern nearly identical to that of reductively hexaPEGylated Hb. Elution position of all these three reductively hexaPEGylated uncrosslinked Hbs is later than that of reductively hexaPEGylated intramolecularly crosslinked Hb (). Accordingly, the PEGylated uncrosslinked carboxymethylated Hbs exist essentially as PEGylated dimers just as reductively hexaPEGylated uncrosslinked Hb under the physiological conditions. Thus, it is clear that avoiding the PEGylation on the α-amino groups of Hb during reductive hexaPEGylation of the molecule by pre-derivatization of these sites does not afford the native Hb-like tetramer stability to reductively hexaPEGylated tetramers.
Figure 2. Size exclusion chromatography analysis of the hexaPEGylated Hbs. hexaPEGylated αα-Hb (1), hexaPEGylated Hb (2), hexaPEGylated [cm-Val-1(α)]2-Hb (3), hexaPEGylated [cm-Val-1(β)]2-Hb (4), were loaded on two HR10/30 Superose 12 columns and eluted with PBS, pH 7.4 at a flow rate of 0.5 ml/min.
![Figure 2. Size exclusion chromatography analysis of the hexaPEGylated Hbs. hexaPEGylated αα-Hb (1), hexaPEGylated Hb (2), hexaPEGylated [cm-Val-1(α)]2-Hb (3), hexaPEGylated [cm-Val-1(β)]2-Hb (4), were loaded on two HR10/30 Superose 12 columns and eluted with PBS, pH 7.4 at a flow rate of 0.5 ml/min.](/cms/asset/935b700d-10d2-4d63-b06d-57e56ad1c0cb/ianb19_a_501756_f0002_b.gif)
Propylation of Val-1(α) of Hb is Adequate to Overcome the HexaPEGylation-induced Promotion of the Dissociation of the Tetramer
[Propyl-Val-1(α)]2-Hb was subjected to PEGylation under the reaction condition that is the same as that for hexaPEGylation of HbA and carboxymethylated Hbs. The elution pattern of the resultant product has been analyzed by size exclusion chromatography and its elution pattern is compared with that of the hexaPEGylated Hb (). [Propyl-Val-1(α)]2-Hb showed an elution pattern comparable to HbA, indicating that propylation of Val-1(α) does not alter the hydrodynamic volume of Hb. On the other hand, the hexaPEGylated [Propyl-Val-1(α)]2-Hb eluted earlier than the hexaPEGylated Hb, and its elution pattern is comparable to that of hexaPEGylated αα-fumarylHb. This establishes that the hexaPEGylated [Propyl-Val-1(α)]2-Hb exhibits a hydrodynamic volume comparable to the hexaPEGylated αα-fumaryl Hb; i.e., avoiding the PEGylation of Val-1(α) attenuates the PEGylation promoted dissociation of the tetramer.
Figure 3. Size exclusion chromatography analysis of the PEGylated proteins. HbA (a), [Propyl-Val-1(α)]2-Hb (b), αα-fumaryl Hb (c), the hexaPEGylated Hb (d), the hexaPEGylated [Propyl-Val-1(α)]2-Hb (e), and the hexaPEGylated αα-fumaryl Hb (f) were loaded on two HR10/30 Superose 12 columns (1 × 31 cm2) and eluted with PBS, pH 7.4 at a flow rate of 0.5 ml/min.
![Figure 3. Size exclusion chromatography analysis of the PEGylated proteins. HbA (a), [Propyl-Val-1(α)]2-Hb (b), αα-fumaryl Hb (c), the hexaPEGylated Hb (d), the hexaPEGylated [Propyl-Val-1(α)]2-Hb (e), and the hexaPEGylated αα-fumaryl Hb (f) were loaded on two HR10/30 Superose 12 columns (1 × 31 cm2) and eluted with PBS, pH 7.4 at a flow rate of 0.5 ml/min.](/cms/asset/49682263-764a-403b-8b76-34cf528e7b68/ianb19_a_501756_f0003_b.gif)
RP-HPLC Analysis of HexaPEGylated Hbs
The hexaPEGylated [Propyl-Val-1(α)]2-Hb has been analyzed by RP-HPLC. As shown in , HbA elutes as a doublet, the two peaks corresponding to the β- and the α-globins (Curve a), respectively, in the order of elution. The peak corresponding to α-globin right-shifted slightly upon propylation of Val-1(α) (Curve b), as the propyl moiety on Val-1(α) increases the overall hydrophobicity of the chain. HexaPEGylation of Hb results in the appearance of three broad peaks. HexaPEGylation of [Propyl-Val-1(α)]2-Hb also led to the appearance of three broad peaks, due to the conjugation of multiple PEG chains. These three peaks were comparable to those for the hexaPEGylated Hb, except that the three peaks were right-shifted.
Figure 4. RP-HPLC analysis of the PEGylated proteins. HPLC analysis of HbA (a), [Propyl-Val-1(α)]2-Hb (b), the hexaPEGylated Hb (c), and the hexaPEGylated [Propyl-al-1(α)]2-Hb (d) was carried out on a Vydac C4 column (4.6 × 250 mm2). The column was eluted with a linear gradient of 35–50% acetonitrile containing 0.1% TFA in 100 min and 50–70% acetonitrile containing 0.1% TFA in 30 min at a flow rate of 1.0 ml/min.
![Figure 4. RP-HPLC analysis of the PEGylated proteins. HPLC analysis of HbA (a), [Propyl-Val-1(α)]2-Hb (b), the hexaPEGylated Hb (c), and the hexaPEGylated [Propyl-al-1(α)]2-Hb (d) was carried out on a Vydac C4 column (4.6 × 250 mm2). The column was eluted with a linear gradient of 35–50% acetonitrile containing 0.1% TFA in 100 min and 50–70% acetonitrile containing 0.1% TFA in 30 min at a flow rate of 1.0 ml/min.](/cms/asset/8d65457d-2843-481e-a05b-6593dc35cd57/ianb19_a_501756_f0004_b.gif)
Dissociation Constant of (Propyl-PEG-5K)6–[Propyl-Val-1(α)]2-Hb is Native-like
The tetramer stabilizing influence of propylation of Val-1(α) of Hb in the reductively hexaPEGylated Hb has quantitated and compared with that of other Hb reductively DiPEGylated at Val-1(β) and hexaPEGylated Hb generated by EAF-PEGylation using maleimide PEG (). The apparent tetramer-dimer dissociation constant (Kd) of the hexaPEGylated [Propyl-Val-1(α)]2-Hb measured by the SEC method is very close to that of that of unmodified Hb. The Kd, of this hexaPEGylated [Propyl-Val-1(α)]2-Hb was around 6.3 μM, whereas the Kd, for HbA was 2.5 μM. The apparent Kd for the EAF-hexaPEGylated Hb [(SP-PEG5K)6Hb] is nearly an order higher than that of Hb; the diPEGylated Hb with propyl PEG-chain on Val-1(β) is comparable to that of the EAF-hexaPEGylated Hb. Thus propylation of Val-1(α) has essentially neutralized the reductive PEGylation-induced promotion of dissociation of tetramer. In doing so, it has neutralized the influence of Propyl PEG-chains on the interdimeric interactions of the PEG-chains.
Figure 5. The tetramer-dimer dissociation constant (Kd) of the PEGylated proteins. HbA (a), the hexaPEGylated [Propyl-Val-1(α)]2-Hb (b), the hexaPEGylated Hb prepared using extension arm facilitated PEGylation (c), and the diPEGylated Hb with PEGylation at Val-1(β) (d) were diluted to a series of Hb concentrations and subjected to two Superose 12 columns (1 × 31 cm2) in series for the Kd measurement.
![Figure 5. The tetramer-dimer dissociation constant (Kd) of the PEGylated proteins. HbA (a), the hexaPEGylated [Propyl-Val-1(α)]2-Hb (b), the hexaPEGylated Hb prepared using extension arm facilitated PEGylation (c), and the diPEGylated Hb with PEGylation at Val-1(β) (d) were diluted to a series of Hb concentrations and subjected to two Superose 12 columns (1 × 31 cm2) in series for the Kd measurement.](/cms/asset/955aa1f0-a72f-4f0e-821e-6a3fb431acf5/ianb19_a_501756_f0005_b.gif)
The dissociation constants for the site-specific chemically modified derivatives of Hbs used for PEGylation are given in . As noted above, of the three derivatives used only propylation of Val-1(α) increases the stability of Hb tetramer by an order of magnitude. Both carboxymethylation and propylation will avoid the modification at the respective sites, but only the propylation of Val-1(α) generates a reductively hexaPEGylated Hb with native tetramer-like stability. This is apparently related to the increase in the stability of (Propyl-PEG5K)6-[Propyl-Val-1(α)]2 Hb is higher than that of [Propyl-PEG5K-Val-1(β)]2 Hb that is (). Since hexaPEGylated propyl Hb is indistinguishable from hexaPEGylated intramolecularly crosslinked Hb, stabilization of the interactions by propylation of Val-1(α) at the αα-end of central cavity plays a dominant role in the stabilization of the reductively PEGylated tetramer. EAF-hexaPEGylated Hb exhibits a stability that is slightly lower than the new reductively PEGylated Hb [Propyl-Val-1(α), and significantly higher than that of hexaPEGylated Hbs generated using other direct PEGylation platforms [thiocarbamoylation [Citation13] or active ester chemistry [Citation12] based PEGylation]. Even at a protein concentration of 0.5 mM, these PEGylated Hbs generated using direct PEGylation platforms exist essentially as PEGylated dimers under physiological conditions.
Functional Properties of HexaPEGylated Hb[Propyl-Val-1(α)] with Native-like Tetramer Stability
The oxygen affinity of the hexaPEGylated [Propyl-Val-1(α)]2-Hb has been compared with that of the reductively hexaPEGylated Hb and hexaPEGylated Hb generated by EAF-PEGylation. As shown in , propylation at Val-1(α) increases the oxygen affinity (i.e., decreases the P50 by nearly 50%) of HbA, however without much influence on the Hill coefficient. Reductive hexaPEGylation of [Propyl-Val-1(α)]2-Hb further decreased the P50 (by another 50%) and this is also accompanied by a reduction in the Hill coefficient. Reductive hexaPEGylation of HbA decreased the P50 (by slightly over 50%) and this is accompanied by a reduction in the Hill coefficient as well.
Table 2. The oxygen affinity of the HexaPEGylated [Propyl-Val-1(α)]2-Hb Sample.
The oxygen affinities of EAF hexaPEGylated Hbs [with and without free Cys-93(β) are also given for comparison. The oxygen affinity of EAF hexaPEGylated Hb (even when Cys-93(β) is PEGylated) is considerably lower than that of hexaPEGylated [Propyl-Val-1(α)]2-Hb and the cooperativity is also better. The very high oxygen affinity of hexaPEGylated [Propyl-Val-1(α)]2-Hb is apparently a consequence of site-specific propylation of Hb as well as the oxygen affinity increasing influence of reductively PEGylated Hb (relative to EAF-PEGylated Hb). The higher P50 of EAF-PEGylated Hb is a combined effect of PEGylation and site-specific PEGylation of Cys-93(β) [Citation9], and oxygen affinity increasing influence is reduced when EAF-PEGylation is targeted exclusively to the terminal amino groups of Hb.
Comparison of Molecular Models of DiPEGylated Hbs with Propyl PEG-5K Chains on Val-1(α) and Val-1(β)
The observation that avoiding the PEGylation neither at Val-1(α) nor at Val-1(β) by carboxymethylation attenuates the PEGylation promoted dissociation of Hb tetramer. This is surprising since there is nearly a thirty-fold difference in the stability of the diPEGylated Hb with PEGylation at these sites, suggesting that the higher stability of [Propyl-PEG-5K (Val-1(β)]2Hb relative to [Propyl-PEG-5K (Val-1(α)]2Hb may be related to a strong influence site-specific PEGylation into the inter dimeric stabilization of the structure. Alternatively, PEGylation-induced weakening of the interdimeric interaction is initiated by the weakening of the interactions at the αα-end of the central cavity of the molecule. Accordingly, in an attempt to understand the differential structural influence of the propyl PEG-chains on Val-1(α) and Val-1(β) on the tetramer stability of the hexaPEGylated tetramer at a molecular level, the models of the two isomeric forms of reductively diPEGylated Hb have been generated. The molecular models depict that the PEG-5K chains conjugated around the αα and the ββ end of the central cavity form loosely organized domains on the surface of Hb (). The orientation of the two PEG domains at the two ends of the central cavity of Hb is distinct, in spite of the fact that the conjugating arm (propyl chain) is very flexible. The differential orientation of the PEG-chains possibly is a consequence of the orientation of the two Val-1(α) vs two Val-1(β) residues in the Hb tetramer and the differential interactions of the PEG-chains with the protein at the two ends of the central cavity. The PEG domains conjugated at Val-1(α) appear to have more conformational freedom. The interdomain distances for the two PEG chains conjugated at the αα-end and at the ββ-end are 66.9 Å and 16.0 Å, respectively. This structural aspect is absent in the diPEGylated Hb with the PEG chains on Val-1(α). This difference apparently impacts the structure of local hydration layer of Hb very distinctly and may, accordingly, impact the tetramer stability of Hb in a very distinct fashion.
Figure 6. Molecular models of [Propyl-PEG5K-Val-1(α)]2-Hb and [Propyl-PEG5K-Val-1(β)]2-Hb. The α- and the β-globin chains are shown in red and blue, respectively. The models were generated as described under Experimental Procedures.
![Figure 6. Molecular models of [Propyl-PEG5K-Val-1(α)]2-Hb and [Propyl-PEG5K-Val-1(β)]2-Hb. The α- and the β-globin chains are shown in red and blue, respectively. The models were generated as described under Experimental Procedures.](/cms/asset/92964cbd-8808-4d60-ba53-a1ec0626c4ad/ianb19_a_501756_f0006_b.jpg)
The differential orientation of the PEG-domains also contributes to a significant difference in the loss of accessible surface area (ASA) (). The loss in ASA is higher in diPEGylated Hb with the PEG-chains conjugated at Val-1(α). As compared to the PEG-chains in the diPEGylated Hb with the PEG-domains on Val-1(β), these interact with one another and result in significant higher loss of ASA of PEG-domains. However, such a contribution is absent in the diPEGylated Hb with the PEG-chains at the αα-end.
Table 3. Loss of Accessible Surface Area on PEGylation of Hb.
Accordingly, we speculate that the inter domain interactions of the Propyl PEG-chains on Val-1(β) at the ββ-end may be acting as “outside the central cavity” pseudo cross-links to strengthen the inter dimeric interactions in this isomeric form of Hb. A “pseudo crosslink effect” leading to an increased stability of the derivatives of Hb tetramer resulting from the electrostatic modifications within the ββ-cleft of Hb been advanced earlier. In the present situation the proposed noncovalent interactions are outside the ββ-cleft. This feature presumably contributes the high stability of the hexaPEGylated propyl Hb. Whether this stabilization is unique to Propyl PEG-chains on the Val-1(β) or thiocarbamoyl phenyl PEG-5K chains [Citation13] as well as propionyl PEG-5K [Citation12] chains also exhibit this interactions that need to be addressed by isolation respective di-PEGylated Hbs and comparative molecular modeling studies.
DISCUSSION
PEGylation of Hb has emerged as a new chemical approach that neutralizes the vasoactivity of acelluar Hb and has energized the research interest in developing PEGylated Hbs as blood substitutes [Citation2]. The delineation of molecular aspects of PEGylation-induced neutralization of the vasoconstrictive activity of Hb has been the subject of considerable debate at this stage. Plasma expander-like properties of PEG-Hb advanced as the primary determinant of the neutralization of the vasoactivity. Nitrite reductase-like activity of PEGylated Hbs [Citation19,Citation20] as well as the PEG-protein mediated induction of e-NOS activity in the endothelium [Citation21] have been advanced as the potential mechanisms, besides the autotransfusion induced by the high COP of the PEG-Hb solutions. The concepts of nitrite reductase-like activity of PEG-Hb and the activation of e-NOS activity are particularly intriguing as they connect the high perfusion seen with the solutions of PEG-proteins with the NO biochemistry of vessel walls.
However, a major limitation of PEGylation of Hb is that it promotes tetramer dissociation and thus limits its application of PEG-Hbs as potential blood substitutes. This was not realized till the demonstration that FPLC of reductively hexaPEGylated Hb is very different as compared to the EAF-hexaPEGyalted Hb. Subsequent studies have now established that EAF PEGylation of Hb is an exception to the PEGylation-induced weakening of the interdimeric interactions of tetrameric Hb, which leaves the interdimeric interactions essentially unperturbed [Citation9]. The understanding of the molecular aspects of PEGylated Hb generated by direct PEGylation platforms, particularly structural factors that promote the tetramer dissociation, is important particularly to pave the way for the design and development of novel approaches to overcome the direct PEGylation promoted dissociation of the Hb tetramers. These studies will lay the foundation for the design of novel PEG-Hbs; the structure of PEG-shell are optimized as oxygen carrying plasma expanders.
PEGylated Hbs are distinct from other Hb derivatives previously designed as blood substitutes. PEGylated Hbs and PEGylated albumins represent a new class of synthetic hybrid biopolymers advanced as semisynthetic plasma expanders. These PEG-proteins consists of two distinct domains/regions that differ significantly in terms of molecular density, and the density of the PEG domain appears to be a function of extent of PEGylation [Citation22] and the molecular size of the PEG. A low-density PEG domain/shell and a high-density protein core form the two distinct regions of this hybrid semi-synthetic biopolymer. The structure of EAF-PEG-Hbs is distinct from that of PEG-Hbs in that the PEG-shell is placed about 8 nm away from the molecular surface of Hb; the interactions of PEG-shell with the protein hydration layer are expected to be lowered in EAF-PEG-Hbs Thus understanding of the influence of PEG domain/shell of EAF-PEG Hb and PEG-Hb on the hydration layer of the protein and its influence on the structured bound water of the central cavity of Hb concomitant impact of this perturbation on structure/function of Hb is an essential prerequisite to undertake the design of novel PEG-Hbs as oxygen-carrying plasma expander.
PEGylation of uncrosslinked Hb with multiple copies of PEG-chains leads to a significant increase in oxygen affinity on the one hand and a lowering of tetramer stability. This suggests the stabilization of the oxyconformation of Hb by PEGylation. All direct PEGylation platforms that we have studied with uncrosslinked Hb [Citation10,Citation12,Citation13] have weakened inter dimeric interactions of the tetramer, and the PEG-Hb adducts exist essentially as dimers under the physiological conditions. On the other hand, the Hb hexaPEGylated using EAF-PEGylation platform exists essentially as tetramers under physiological conditions. Besides, when the EAF PEGylation is targeted exclusively at the ε-amino groups of Hb, the EAF hexaPEGylated Hb generated exhibits a tetramer stability nearly identical to that of uncrosslinked Hb [Citation9]. Apparently, the extension arms engineered between the PEG-chains and protein essentially attenuate the influence of PEGylation on the weakening of the interdimeric interactions. Consistent with this is the results of our recent study on the development of an extension arm version of acylation chemistry-based direct PEGylation [Citation15]. Accordingly, insertion of extension arms between the functional groups of proteins and the PEG-chains has been advanced as a chemical strategy to overcome the PEGylation-promoted dissociation of Hb tetramer in the design of PEG-Hb as the oxygen-carrying plasma expander.
The physiological complication of lowered tetramer stability of PEGylated Hbs has not been yet addressed much so far. This aspect of the structure of PEGylated Hbs can increase the vascular leakage on the one hand and autoxidation-mediated in vivo toxicity of PEGylated Hbs [Citation23] on the other, but the nephrotoxicity is unlikely to be seen with this as the PEGylated dimers still exhibit very high hydrodynamic volume and are unlikely to filter through glomular filtration. Ann Baldwin and her associates have noted vascular leakage of Enzon PEGylated bovine Hb, direct PEGylation promoted dissociation of the PEGylated tetramer may be the factor responsible for this molecular event.
The promotion of the dissociation of tetramer seen on direct PEGylation of Hb could be a consequence of site-specific PEGylation of Hb or a global effect of PEGylation (i.e, a multisite effect) on the interdimeric interactions of Hb. In the case of reductive hexaPEGylation, the site-specific PEGylation-mediated weakening of interdimeric interactions has been established. For example, the propyl PEG-chains on the amino terminus of α- and β-chains lower the tetramer stability, but the propyl PEG-chains on Val-1(α) appear to contribute significantly more towards the PEGylation-induced promotion of tetramer dissociation and Hb diPEGylated on Val-1(α) exists essentially as PEGylated dimers. In this study we have compared the propensity of avoiding the PEGylation of Val-1(α) with and without increasing the stability of the tetramer to overcome the promotion of the tetramer dissociation seen on reductive PEGylation.
The reductive PEGylation-induced promotion of tetramer dissociation is not attenuated or neutralized by avoiding the PEGylation of Hb at Val-1(α) or even at Val-1(β) or of both sites, which is consistent with the concept that PEGylation-induced destabilization of the tetrameric structure of Hb is a multi-site effect. If avoiding the PEGylation at specific site(s) neutralizes the promotion of tetramer dissociation seen on reductive PEGylation, it will be the confirmation that promotion of the tetramer dissociation as a direct consequence of site-specific PEGylation-induced destabilization of the quaternary structure of Hb. The reductive hexaPEGylation of site-specifically carboxymethylated Hbs failed to overcome the PEGylation-induced tetramer dissociation. PEGylation of the carboxymethylated Hb at other sites is more than adequate to perturb the protein hydration layer and promote the tetramer dissociation just as with unmodified Hb, i.e. promotion of tetramer dissociation on reductive PEGylation is a multi-site effect.
On the other hand, avoiding the PEGylation on Val-1(α) by propylation, the reductive PEGylation-promoted tetramer dissociation nearly completely neutralized. The propylation of Val-1(α) of Hb results in the strengthening of the interdimeric interactions tetramer by nearly an order of magnitude besides avoiding the PEGylation at this site. This double-edged approach results in the neutralization of reductive PEGylation-induced promotion of the dissociation of the tetramer.
The molecular aspects of the stabilization of the tetrameric structure of Hb by the propylation of Val-1(α) is not readily apparent at this stage. Both propylation and carboxymethylation are expected to lower the pKa of the α-amino group. The propyl side chain is more hydrophobic relative to the carboxymethyl chain. Accordingly, we speculate increasing the hydrophobicity of the micro-environment at the αα-end of the central cavity of Hb facilitates the strengthening of the interdimeric interactions of the tetramer. This may be a consequence of the increased hydrophobicity-induced stabilization of the αα-end of Hb. This would suggest that if longer alkyl chains that can increase the hydrophobicity better than propyl chains are used to reductively alkylate Val-1(α), can induce higher stability to Hb, and may be better reagents to overcome the reductive PEGylation-induced dissociation of the tetramer.
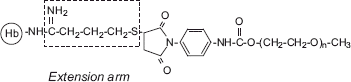
It may be noted that EAF PEGylation of Hb () does not exhibit PEGylation-induced promotion of tetramer dissociation [Citation9]. Apparently, this been achieved by minimizing the influence of PEGylation on the central cavity of Hb by the engineering of extension arms between the PEG-chains and protein functional groups (). The presence of extension arms presumably modulates the level of perturbation of the hydration layer of Hb by the PEG-chains. We are now actively pursuing the influence of the length of the extension arm in such modulation as a way of minimizing the PEGylation-induced weakening of the interdimeric interactions. We had earlier advocated the development of the extension arm chemistry version of direct PEGylation platforms as a novel approach to overcome the promotion of tetramer dissociation associated with PEGylation [Citation15]; any potential advantages of site selectivity of PEGylation chemistry could be conserved in developing such extension arm chemistry versions by using the same chemistry to introduce the extension arms onto the protein.
Figure 7. Schematic representation of the extension arms sandwiched between the PEG-chains and side chains of Hb in the EAF-PEGylated Hb. Note the extension arm between the amino group and succinimidyl phenyl PEG chains, which helps to attenuate the influence of the PEG-chains on the interdimeric interactions (central cavity interactions) of Hb tetramer.
The present study advances an alternate approach, i.e. an alternate approach, namely to increase the stability of interdimeric interactions of the Hb tetramer rather than minimizing the perturbation of the interdimeric interactions by PEGylation. This is achieved through site-specific chemical modification of the molecule. It remains to be seen whether the latter approach can also stabilize the hexaPEGylated Hb generated by acylation chemistry-based PEGylation (using succinimido propionyl PEG) just as the EAF version of this PEGylation platform has achieved. A number of site-directed mutagenic approaches that increase the stability of the tetramer have been developed. Many central cavity mutants with low oxygen affinity have been designed by Ho and his colleagues [Citation24], which conserve the deoxy-like structure in the oxy conformation that [Citation25] could possibly attenuate the direct PEGylation induced weakening of the interdimeric interactions. It is important to establish whether a simple stabilization of the interactions at the αα-end of the molecule is achieved that can be achieved by site-directed mutagenesis is enough, i. e. without avoiding the PEGylation of Val-1(α)] before we can undertake this new chemical approach with recombinant Hbs with enhanced stabilization of the tetrameric structure.
There are two PEGylated uncrosslinked Hbs with high oxygen affinity in clinical trial, namely MP4 and the Enzon PEGylated Hb. The Enzon PEGylated Hb is a decaPEGylated species using succinimido carbonate of PEG-5K; direct conjugation of bovine Hb with PEG5K chains occurs through urethane linkage. Some of the earlier studies have suggested that the Enzon PEGylated Hb leaks through vasculature and this leads to in vivo toxicity [26]. The capillary leakage of Enzon PEGylated Hb could be due to the weakened interdimeric interactions of uncrosslinked PEG Hb. Extensive preclinical and clinical studies established that MP4 is essentially nontoxic [Citation6–7], possibly due to the fact that the EAF PEGylation has marginal influence on the interdimeric interfaces. The lower stability of these PEGylated tetramer vs MP4 may be one if not the only factor that might have played a role in this difference with respect to the lack of in vivo toxicity of the two PEGylated Hbs.
In designing the second-generation low oxygen affinity EAF PEGylated crosslinked Hbs, we have been trying to integrate our chemical strategy to generate vasoinactive Hbs, i.e., PEGylation-induced neutralization of vasoactivity of Hb, for overcoming the vasoactivity of previously designed low oxygen affinity crosslinked or oligomerizes Hb (references). A new opportunity to develop PEGylated Hbs without intramolecular crosslinking is presented by our current studies on reductive propylation of [Propyl-Val-1(α)]2 Hb and also our recent studies of extension arm facilitated PEGylation targeted exclusively to the amino groups of Hb [Citation9]. Thus these studies have paved the way for developing PEG-Hbs without the stabilization of the Hb by intermolecular crosslinking, and undertake the studies on correlation of tissue oxygenation by PEG-Hbs as a function of oxygen affinity and map the influence of intramolecular crosslinking on tissue oxygenation.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Intaglietta, M. (1997). Ann. Biomed. Eng. 25: 593–603.
- Winslow, R. M., Gonzales, A., Gonzales, M. L., Magde, M., McCarthy, M., Rohlfs, R. J. Vandegriff, K. D. (1998). J. Appl. Physiol. 85:993–1003.
- Winslow, R. M. (2000). Adv. Drug Del. Rev. 40: 131–142.
- Li, D., Manjula, B.N., Acharya, A.S. (2006). Protein J. 25: 263–274.
- Manjula, B. N., Tsai, A. G., Intaglietta, M., Tsai, C-H., Ho, C., Smith, P. K., Perumalsamy, K., Kanika, N. D., Friedman, J. M., Acharya, A. S. (2005). Protein J. 42: 133–146.
- Vandegriff, K.D., Winslow, R.M. (2009). Artif. Organs 33: 133–138.
- Olofsson, C., Ahl, T., Johansson, T., Larsson, S., Nellgård, P., Ponzer, S., Fagrell, B., Przybelski, R., Keipert, P., Winslow, N., Winslow, R.M. (2006). Anesthesiology 105: 1153–1163.
- Manjula, B. N., Tsai, A., Upadhya, R., Perumalsamy, K., Smith, P. K., Malavalli, A., Vandegriff, K. D., Winslow, R. M., Intaglietta, M., Prabhakaran, M., Friedman, J. M., Acharya, A. S. (2003). Bioconjugate Chem. 14: 464–472.
- Li, D., Hu, T., Manjula, B.N., Acharya, S.A. (2009). Bioconjug Chem. 20(11): 2062–2070.
- Hu, T., Prabhkaran, M., Acharya, S.A., Manjula, B.N. (2005). Biochem. J. 392: 555–564.
- Hu, T., Manjula, B. N., Li, D., Brenowitz, M., Acharya, S.A. (2007). Biochem. J. 402: 143–151.
- Li, D., Hu, T., Manjula, B.N., Acharya, S.A. (2008). Biochim Biophys Acta. 1784(10): 1395–401.
- Meng, F., Manjula, B.N., Tsai, A.G., Cabrales, P., Intaglietta, M., Smith, P.K., Prabhakaran, M., Acharya, S.A. (2009). Protein J. 28(5): 199–212.
- Hu, T., Li, D., Manjula, B. N., Brenowitz, M., Prabhakaran, M., Acharya, S.A. (2009). Biochemistry 48: 608–616.
- Ananda, K., Acharya, S.A. (2008). Artif Cells Blood Substit Immobil Biotechnol. 36: 499–512.
- Manjula, B.N., Acharya, A.S. (2003). Methods Mol. Med. 82:31–47.
- DiDonato, A., Fantl, W.J., Acharya, A.S., Manning, J.M. (1983). J Biol Chem. 258(19): 11890–5.
- Manning, L.R., Jenkins, W.T., Hess, J.R., Vandegriff, K., Winslow, T.M., Manning, J.M. (1996). Protein Sci. 5: 775–781.
- Lui, F.E., Kluger, R. (2009). Biochemistry 48(50): 11912–9.
- Martini, J., Cabrales, P., Acharya, S.A., Intaglietta, M., Tsai, A.G. (2008). Crit Care 12(2): R54.
- Meng, F., Manjula, B.N., Smith, P.K., Acharya, S.A. (2008). Bioconjug Chem. 19(7): 1352–60.
- Hu, T., Li, D., Manjula, B. N., Acharya, S.A. (2008). Biochemistry 47: 10981–10990.
- Tsai, C.H., Fang, T.Y., Ho, N.T., Ho, C. (2000). Biochemistry 39(45): 13719–29.
- Acharya, S.A., Malavalli, A., Peterson, E., Sun, P.D., Ho, C., Prabhakaran, M., Arnone, A., Manjula, B.N., Friedman, J.M. (2003). J Protein Chem. 22(3): 221–30.
- Baldwin, A.L. (1997). Advances in Blood Substitutes: Industrial Opportunities and Medical Challenges, R. M. Winslow, K. D. Vandergriff, and M. Intaglietta, Birkhaüser, Boston, 19–37.