Abstract
Various types of porous resin adsorbents based on polystyrene, agarose, and cellulose as matrixes coupling with DNA, amino acids and other biological active molecules as ligands were extensively studied in China. Molecular recognition between the ligand and pathogenic molecule was investigated. Several commercialized products are now widely used in hospitals all over China. Whole blood hemoperfusion is used to treat patients suffering from autoimmune diseases, uremia acute intoxication, and hyperbilirubinemia. Clinical performances of hundreds and thousands of patients treated by whole blood sorption therapy show that the therapy is safe, efficient, and cost-effective.
INTRODUCTION
Various methods for the removal of pathogenic toxins in blood purification were extensively studied by scientists all over the world. Among the new technologies investigated, a major trend of apheresis equipment developed in 2001 was the introduction of many specific adsorbent columns that can not only remove exogenous and endogenous small molecular weight toxic molecules but also adsorb middle molecules and macromolecular toxins. By linking bioactive ligands to the adsorbents, low-density lipoproteins, cholesterol, β2-microglobulin, endotoxin, mediators in sepsis, rheumatoid factors, antibodies in systemic lupus erythematosus (SLE) immune-complexes and many other pathological macromolecules can be easily removed. We are now in the era of sorption apheresis.
The history of research and development of adsorbents in China goes back to the late 1970s when Dr. T.M.S. Chang from Canada first visited China and gave a series of lectures on artificial cells and organs [Citation1–4]. Albumin can bind tightly to the ultrathin collodion membrane of adsorbent artificial cells, and was initially used to increase the blood compatibility of the collodion-coated adsorbent artificial cells for hemoperfusion [Citation5]. This albumin-collodion coating has also been applied to synthetic immunosorbents, resulting in blood-compatible synthetic blood group immunosorbents [Citation6]. Terman et al. found that this albumin-coated collodion-activated charcoal can also effectively remove antibodies to albumin in animal studies and applied this principle of adsorbing antigens to the collodion-coated charcoal for the specific removal of other antibody [Citation7]. His exciting lectures stimulated the interest of the Chinese scientists to be engaged in biomaterials, especially in artificial organs. A research team was then established at Nankai University headed by Dr. Y.T. Yu, who later visited Chang's lab (Artificial Cells and Organs Research Center) at McGill University in 1980 as a visiting scholar researching encapsulated multi-enzymes converting urea (from uremia patients) into amino acids [Citation8,Citation9]. After his return, the group was determined to investigate the possibility of using porous resins, linked with various bioactive ligands, as adsorbents for the removal of pathogenic molecules of different molecular weights by hemoperfusion. Their strategy for developing resin adsorbents was: 1) safety; 2) high efficiency; and 3) cost-effective. In order to reduce the burden of healthcare agencies so that a large number of patients could be treated and improve their living quality, the Nankai group used the whole blood perfusion method and tailor-made various kinds of resins to achieve high performance (efficiency 90%) in blood purification. This paper mainly focuses on the development, commercialization, and clinical performance of various kinds of resins (polystyrene, cellulose-based adsorbents) for the sorption therapy of diseases (SLE, acute intoxication) with low or no medication efficiency.
POLYSTYRENE-BASED ADSORBENT
Carbonized Resin Immobilized DNA
In 1983, carbonized resin beads were made by the Nankai group from a styrene copolymer by pyrolysis at high temperature. DNA was then coated on the resin with a collodion solution to form an immuno-adsorbent. Whole blood from SLE patients was perfused through a cartridge containing the immuno-adsorbent.
Clinical reports of 23 patients showed that the pathogenic anti-DNA antibody was removed at an average of 56%, immuno-complexes were sharply reduced, and symptoms were relieved. After perfusion, no significant changes in hemoglobin, leukocyte count, total protein, and electrolytes were observed. Platelet depletion was in the permissible range. Whole blood hemoperfusion with the immuno-adsorbent was effective (90%) for treatment of SLE and the commercialized cartridges are now used in hospitals all over China [Citation10–18].
Large Pore-size Porous Resin
Various macroporous resins were tailor-made by suspension polymerization of styrene and divinyl benzene with various amounts of pore-forming materials at Nankai University. Resins with different pore diameters and surface areas were studied for the adsorption capacity of unconjugated bilirubin ().
Table 1. Various surface area and pore size of resins versus their adsorption capacities for bilirubin.
From data shown above we can clearly see that NK-110 resin having a pore size of 160Å and surface area of 500 m2/g showed the highest adsorption capacity for bilirubin. This is because the large size of the unconjugated bilirubin molecule could diffuse freely through the large pores of the resin. From the above findings, a slightly modified resin was commercialized and successfully used in clinics for the treatment of hyperbilirubinemia patients in whole blood hemoperfusion [Citation19].
High Surface Area Resin
High surface area of 1076 m2/g porous resin was made from polystyrene by the Nankai group [Citation20]. The bead-type porous resin has a high adsorption capacity for small molecules such as drugs and chemicals ().
Table 2. Physical properties and adsorption capacities of the resin.
The physiological and blood-compatible properties of the adsorbent were approved by SFDA (Chinese FDA). The resin was produced on an industrial scale and used clinically for detoxification of acute intoxicated patients in hospitals all over China.
CELLULOSE-BASED ADSORBENT
Cellulose is a natural biomaterial that is biodegradable and has good biocompatibility properties. Various adsorbents based on cellulose as matrixes were extensively studied (). An adsorbent for the removal of bacterial endotoxins was developed by immobilizing lysine covalently onto cellulose beads [Citation21]. In vivo studies showed that the mean blood endotoxin concentration in rabbits was reduced significantly from 5.56 ± 0.54 EU/ml to 0.41 ± 0.26 EU/ml after perfusion without significant side-effects. It has a high potential of clinical application for treatment of endotoxemia patients [Citation22].
Table 3. Cellulose-based adsorbents.
Another approach showed cellulose beads linked with phenylalanine ligand can remove rheumatoid factors [Citation23]. In vivo results show that the adsorbent holds promise as a highly effective and safe adsorbent in clinical therapy for rheumatoid arthritis patients by hemoperfusion [Citation24].
It is well known that dendrimers have a large number of terminal functional groups. A synthetic approach involved arching of poly(amidoamine) (PAMAM) onto cellulose beads by reaction with epichlorohydrin was conducted [Citation25]. The adsorbent showed a high amount of ligands linked and an enhanced adsorption percentage for rheumatoid factors [Citation26].
In order to remove the anti-DNA antibody from the blood of patients with systematic lupus erythematosus (SLE), DNA immuno-adsorbent was prepared by coupling calf thymus DNA to epichlorohydrin-activated cellulose beads. In vitro adsorption tests showed that the DNA immuno-adsorbent could remove 40%–70% of anti-DNA antibody from the plasma (incubation of 1.0 ml of adsorbent with 3.0 ml of plasma) [Citation27]. In the circulation tests, 30 ml of plasma was circulated through a column containing 3 ml of adsorbent. The maximum decrease of anti-DNA level was 80% after 60 min. The high adsorption capacity and rate suggest the immuno-adsorbent may be used for clinical treatment [Citation28].
Immuno-adsorbents for removing pathogenic antibodies of myasthenia gravis were prepared by coupling tryptophan onto cellulose beads. In vivo hemoperfusion experimental results showed that the removal of acetylcholine receptor antibodies was 25% [Citation29,Citation30].
Hyperlipidemia is one of the high-risk factors for the development of atherosclerosis. In order to remove high levels of low density lipoprotein (LDL), especially in familial hypercholesterolemia patients, cellulose-based LDL adsorbent with phosphate functional groups was prepared. The adsorption capability was 3.12 mg/ml, which proved the phosphate adsorbent had high adsorption capability and excellent adsorption selectivity. The autoclave and storage test showed the adsorbent was stable [Citation31]. Cellulose-based LDL adsorbent with taurine functional group and PAMAM spacer also showed high adsorption capacity for LDL [Citation32].
In order to enhance the adsorption capacity of the developed adsorbent, a new amphiphilic adsorbent for the removal of LDL was prepared by introducing cholesterol and sulfonic groups onto cellulose beads [Citation33,Citation34]. In vitro studies showed that the amphiphilic adsorbent had a high adsorption capacity for LDL without significantly adsorbing high-density lipoprotein. The adsorption capacity of the adsorbent for the removal of LDL, total cholesterol, and triglyceride was 1.916, 2.132, 1.349 mg/ml, respectively. After 2 h hemoperfusion in hyperlipidemia rabbits, the LDL levels decreased from 3.619 ± 0.354 to 0.724 ± 0.07 mmol/l, demonstrating that the adsorbent could effectively remove LDL [Citation35].
POLY (VINYL ALCOHOL)-BASED ADSORBENT
Polyvinyl alcohol has been widely used in biomedical applications and has good blood compatibility. Macroporous polyvinyl alcohol microspheres were prepared and L-phenylalanine was immobilized as the ligand. The adsorbent could remove high levels of rheumatoid factors (RFs) from the blood of rheumatoid arthritis patients at a capacity of 750 IU/ml [Citation36].
A series of bilirubin adsorbents was generated by linking trimethylamine, triethylamine, and 1,6-hexanediamine on macroporous polyvinyl alcohol beads. The adsorption efficiencies of bilirubin reached 80% [Citation37,Citation38].
Recently, adsorbents for sepsis were prepared by incorporating hexane diamine onto macroporous polyvinyl alcohol microspheres. The adsorbents had high adsorption efficiencies for TNF-α (72%), IL-6 (91%) and endotoxin (96%).
PMMA-BASED ADSORBENT
Various adsorbents have been investigated for removing endotoxin from protein solutions by polymethylmethacrylate (PMMA)-based adsorbents coupling with dimethylamine ligand. Experimental results indicated that the adsorbent having a hydroxyl group at the beta-site of the ligand had an enhanced adsorption capacity for endotoxin. The computer simulation showed that the hydroxyl group at the beta-site could form hydrogen bonds with endotoxin; as a result, an octatomic ring was formed. The spacer of the adsorbent and the long alkyl chain of endotoxin were located on the same side of the octatomic ring. In this situation, electrostatic interaction, hydrogen bond, cooperative effect of octatomic ring, and hydrophobic intermolecular interaction effected simultaneously. The combination of endotoxin with adsorbent was tight and adsorption capacity was effectively increased [Citation39].
ADSORBENTS WITH OTHER MATRIXES AND LIGANDS
Chitosan-based adsorbent was also studied for the removal of LDL adsorption with a capacity of 2.72 mg/ml [Citation31].
Agar or agarose was covalently linked on heat- aggregated human IgG as a ligand for the removal of rheumatoid factors. The adsorbent capacities for IgMRF, IgGRF, IgARF were 3400, 2240, and 2400 IU/g, respectively [Citation40].
MOLECULAR RECOGNITION MECHANISM BETWEEN LIGAND AND TOXIC MOLECULE
In general, there are four types of interacting forces involved between the ligand and the toxic molecule, i.e. electrostatic interaction, hydrophobic interaction, hydrogen bonding, and Van der Waals’ force. The authors found that electrostatic force plays an important role and enhances the adsorption capacity drastically in the case where the toxic molecule to be removed has a high polarity [Citation41,Citation42]. Hydrophobic interaction is crucially important in the case where the ligand and toxic molecule are non-polar [Citation43]. Antigen-antibody interaction contributes to the specificity between the molecular recognition. Computer modeling was also used to study the reaction mechanism [Citation40,Citation44]. These findings attribute to the designing of more efficient adsorbents.
SPACER EFFECT
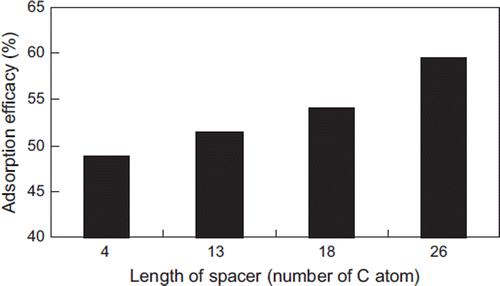
The spacers have a significant effect on the adsorption property of resin adsorbents. They can reduce the steric hindrance between the ligand and the large toxic molecule, resulting in an increase of adsorption capacity of the adsorbent. Different spacers have an obvious effect on the adsorption properties of adsorbents. When IgG was coupled by N,N’-Carbonyldiimidazole (CDI), the immuno-adsorbent had an adsorption efficiency of 30∼35% for IgA in vitro experiments [Citation45]. When it was coupled by glycidyl 1,4-butanediol, the adsorption efficiency was 60%, which was much higher than that of the CDI coupling method [Citation46]. And when it was coupled by epoxy chloropropane and glutaraldehyde, the adsorbent efficiency reached 74% [Citation47].
The length of a spacer (number of carbon atoms) influences significantly the adsorption capacity of a resin. As shown in , the adsorption capacity increases as the number of carbon atoms increases (about 12%) [Citation26].
SCALE-UP PRODUCTION AND CLINICAL PERFORMANCE
In China, there are 4–5 companies engaged in the production of adsorbents on an industrial scale, of which Jian Fan Biological Science and Technology Company in Zhu Hai is the largest. From 2001 they have developed three products for the therapy of SLE, hyperbilirubinemia, uremia, and various acute intoxication patients with the technology provided by the Nankai group. Statistics studies for the last three years showed that more than 200,000 cartridges were produced and used in 2,300 hospitals in China. The total productive value reached 150 million RMB (∼ 24 million USD).
Whole blood sorption therapy was performed on hundreds and thousands of patients suffering autoimmune diseases, various cases of acute intoxication such as suicide, drug overdose, accidental contact with organo-phosphorous insecticides, snake venom, and tetrodotoxin were successfully treated. Symptoms were relieved, lives were saved, no significant side-effects occurred, and an efficiency of 90% was achieved. More important is that whole blood perfusion with locally produced adsorbents is cost-effective. A cartridge of a few hundred USD is available, so a large number of patients are able to afford it, which at the same time drastically reduces the high medical burden of healthcare agencies. Statistic studies from Jian-Fan Biological Science and Technology Company showed that for the past three years a total of about 200,000 patients were successfully treated by whole blood perfusion, thus up-grading the quality of their living standard and prolonging their survival rates.
CONCLUDING REMARKS
Thirty years of research and development in adsorbents by whole blood purification in China have paved a path for the treatment of patients with autoimmune diseases and acute intoxication cases. Clinical performances have proved that it was safe, efficient, and cost-effective. At this point, we are grateful to our state and local governmental departments, who have financially supported the Nankai research group continuously. We want to thank Dr. T.M.S Chang for his kind instructions and upgrading Chinese students and visiting scholars. Many thanks also go to Dr. H. Klinkmann for his continuous support of Nankai University via INFA.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Chang, T.M.S. (1964). Semipermeable microcapsules. Science 146:524–525.
- Chang, T.M.S. (1972). Artificial Cells. Monograph. Charles C. Thomas, Springfield, IL (full text available at http:\\www.artcell.mcgill.ca).
- Chang, T.M.S. (1975). Microencapsulated adsorbent hemoperfusion for uremia, intoxication and hepatic failure. Kidney Int. 7:S387–S392.
- Chang, T.M.S. (1976). Biodegradable semipermeable microcapsules containing enzymes, hormones, vaccines and other biologicals. J. Bioengineering 1:25–32.
- Chang, T.M.S., Gonda, A., Dirks, J.H., Malave, N. (1969). Removal of endogenous and exogenous toxins by a micro encapsulated adsorbent. Can J Physiol Pharmacol. 47: 1043–1045.
- Chang, T.M.S. (1980). Blood compatible coating of synthetic immunoadsorbents. Trans Am Soc Artif Intern Organs. 26:546–549.
- Terman, D.S., Tavel, T., Rade, M.R., Petty, D., Buffaloe, G. (1977). Specific removal of antibody by extracorporeal circulation over antigen immobilized in collodion-charcoal. Clin Exp Immunol. 28:180–188.
- Yu, Y.T., Chang, T.M.S. (1981). Ultrathin lipid-polymer membrane microcapsules containing multienzymes, cofactors and substrates for multi-step enzyme reactions. FEBS Letters, 125:94–96.
- Yu, Y.T., Chang, T.M.S. (1982). Immobilization of multi-enzymes and cofactors within lipid-polyamide membrane microcapsules for the multi-step conversion of lipophilic and lipophobic substrates. Enzyme Microb Technol, 4: 327–331.
- Yang, Y., Yu, Y.T., Song, J.C., Qian, S.C., Quan, W.L., Shao, X.H., Cui, R.H., Wang, J.Z. (1987). Preparation and clinical trial of a new DNA immune adsorbent for hemoperfusion in systemic lupus erythematosus (SLE) therapy. Artif Organs, 11:334.
- Yang, Y., Yu, Y.T., Song, J.C., Qian, S.C., Quan, W.L., Shao, X.H., Cui, R.H., Wang, J.Z. (1988). A new DNA immune adsorbent for hemoperfusion in SLE therapy – a clinical trial. Artif Organs, 12:444–448.
- Gao, C.L., Li, B.A., Chen, C.Z., Yu, Y.T. (1995). Clinical trials of immunoadsorbent in systemic lupus erythematosus therapy. Artif Organs, 19:468–469.
- Kong, D.L., Chen, C.Z., Lin, E.F., Yu, Y.T. (1998). Clinical trials of type I and in vitro studies of type II immunoadsorbents for systemic lupus erythematosus therapy. Artif Organs, 22:644–650.
- Yu, Y.T., Fu, C.X., Kong, D.L., Chen, C.Z., Lin, E.F. (1998). Immunoadsorbents for systemic lupus erythematosus and rheumatoid arthritis therapy. Artif Cells, Blood Substit Immobilization Biotechnol, 26:94.
- Zhu, B., Iwata, H., Kong, D.L., Yu, Y.T., Kato, K., Ikada, Y. (1999). Preparation of DNA-immobilized immunoadsorbent for treatment of systemic lupus erythematosus. J Biomater Sci-Polym Ed. 10:341–350.
- Yu, Y.T., Chen, C.Z., Kong., D.L. (2001). Method for Preparing a Carbonized Resin DNA Immunoadsorbent. US 6262172.
- Yu, Y.T., Chen, C.Z., Kong, D.L. (1998). Method for Preparing a Carbonized Resin DNA Immunoadsorbent. CN 98102355X.
- Yu, Y.T., Chen, C.Z., Kong., D.L. (1998). Bead Type Cellulose Based DNA Immunoadsorbent. CN 98102354.1.
- Tong, M.R., Chen, C.Z., Liu, R.X., Yu, Y.T. (1983). Removal of unconjugated bilirubin by high adsorption capacity nonionic polymeric adsorbent NK-110. Proceeding of the V International Symposium on Hemoperfusion and Artificial Organs, 379–386. China Academic Publishers, Tianjin, China.
- Chen, C.Z., Zhang, Q.X., Yu, Y.T. . (1983). New uncoated adsorbent NK-107 of high surface area for treatment of acute intoxication. Proceeding of the V International Symposium on Hemoperfusion and Artificial Organs, 350–357. China Academic Publishers, Tianjin, China.
- Fang, H., Wei, J., Yu, Y.T. (2004). Studies on adsorption performance of affinity adsorbent for bacterial endotoxins. Chem J Chin Univ-Chin, 25:1056–1059.
- Fang, H., Wei, J., Yu, Y.T. (2004). In vivo studies of endotoxin removal by lysine-cellulose adsorbents. Biomaterials, 25:5433–5440.
- Dong, Q.W., Wang, L.Y., Yu, Y.T., Wu, C. (2006). Preparation and adsorption capability of several adsorbents for rheumatoid arthritis. Acta Scientiarum Naturalium Universitatis Nankaiensis, 39:79–85.
- Wang, Y.J., Yu, Y.T., Li, Z.J. (2005). In vitro and in vivo evaluation of amino acid-functionalized cellulose beads for whole blood hemoperfusion. Key Eng Mat, 288–289: 393–396.
- Wang, Y.J., Liu, J.F., Duo, J., Yu, Y.T. (2005). New method for the preparation of adsorbent with high adsorption capacity. Chin Sci Bull, 50:2432–2435.
- Wang, Y.J., Wang, L.Y., Yu, Y.T. (2005). Study on the rheumatoid arthritis adsorbents and the influence of spacers. Chin J Biomed Eng, 24:486–491.
- Kong, D.L., Dai, J., Chen, C.Z., Yu, Y.T. (2000). Coupling of DNA to bead type cellulose as immunoadsorbent. Chem J Chin Univ-Chin, 21:1848–1851.
- Kong, D.L., Schuett, W., Dai, J., Kunkel, S., Holtz, M., Yamada, R., Yu, Y.T., Klinkmann, H. (2002). Development of cellulose DNA immunoadsorbent. Artif Organs, 26: 200–208.
- Yan, W.R., Yu, Y.T., Yang, L., Zhang, W.H., Miao, P.C. (2002). Studies on new immunoadsorbent for myasthenia gravis. Chem J Chin Univ-Chin, 23:1887–1890.
- Yang, L., Cheng, Y., Yan, W.R., Yu, Y.T. (2004). Extracorporcal whole blood immunoadsorption of autoimmune myasthenia gravis by cellulose tryptophan adsorbent. Artif Cells Blood Substit Biotechnol, 32:519–528.
- Wang, T., Wang, W.C., Wang, L.Y., Wei, D., Wang, S.Q., Yu, Y.T., Kong, D.L. (2008). Phosphate-based affinity adsorbents for the removal of low density lipoprotein: preparation and in-vitro adsorption capability test. Chin J Biomed Eng, 27:132–136.
- Yuan, Y., Wang Y.M., Yu, Y.T. (2008). Study of the LDL adsorbent with dendrimer PAMAM as the spacer. Ion Exchange & Adsorption, 24:1–9.
- Wang, S.Q., Yu, Y.T., Cui, T., Cheng, Y. (2002). Cellulose amphiphilic adsorbent for removal of low density lipoprotein. Artif Cells Blood Substit Biotechnol, 30:285–292.
- Cui, T., Cheng, Y., Wang, S.Q., Yu, Y.T. (2005). Study on the lowering-lipid capability of a novel LDL-adsorbent. Chin Biomed Eng, 24:50–53.
- Cheng, Y., Wang, S.Q., Yu, Y.T., Yuan, Y. (2003). In vitro in vivo studies of a new amphiphilic adsorbent for removal of low density lipoprotein. Biomaterials, 24:2189–2194.
- Wang, W.C., Hu, Y.H., Ou, L.L., Kong, D.L., Yu, Y.T. (2009). Preparation and evaluation of an adsorbent for rheumatoid factors. Chin J Biomed Eng, 28:561–566.
- Wang, W.C., Zhang, S.N., Hu, Y.H., Xie, H., Ou, L.L., Yu, Y.T., Kong, D.L., Gu, H.Q. (2008). Preparation of aminated macroporous polyvinyl alcohol resins and evaluation for bilirubin adsorption. Chin J Biomed Eng (Eng Ed), 17: 93–100.
- Zhang, S.N., Wang, W.C., Hu, Y.H., Xie, H., Ou, LL., Yu, Y.T., Kong, D.L., Gu, H.Q. (2005). Preparation of aminated macroporous polyvinyl alcohol resins and evaluation for bilirubin adsorption. Chinese J Dial & Artie Organs, 19:1–5.
- Yuan, Z., Yu, M., Li, J.H., Hou, G.H., Wang, H.Y. (2005). Endotoxin adsorbent using dimethylamine ligands. Biomaterials, 26:2741–2747.
- Fu, C.X., Yu, Y.T., Chen, C.Z. (2000). A novel immunoadsorbent for rheumatoid arthritis therapy. Art Cells Blood Subs and Immob Biotech., 28:409–414.
- Wang, H.M., Chen, C.Z., Tong, M.R., Zheng, J.T., Yu, Y.T. (1989). Synthesis of macroporous poly-α-vinylpyridine resins and its removal of unconjugated bilirubin. Acta Polym Sin, 171–176.
- Wang, L.Y., Lu, J., Yu, Y.T. (2004). Interaction of rheumatoid factor with immobilized ss-DNA. Chem Res Chin Univ, 20:795–800.
- Cao, N.N., Yu, Y.T., Wang, M.Y., Wang, L.Y., Chen, C.Z. (2002). The interaction of human lipoprotein with cholesterol modified dextran in serum. Chem J Chin Univ-Chin, 23:1524–1528.
- Yan, W.R., Yu, Y.T., Dong, N. (2003). Computer aided study of mechanism of immunoadsorbent for myasthenia gravis. Chin Chem Lett, 14:693–696.
- Chen, J.J., Chu, J.Q., Kong, D.L., Yu, Y.T., Zhu, B.R., Liu, J.D. (2002). In vitro studies of the immunoadsorbent for removal of IgA in IgA-nephropathy (I). Ion Exchange & Adsorption, 18:369–373.
- Chu, J.Q., Yu, Y.T., Zhang, W.H., Chen, J.J., Zhu, B.R., Liu, J.D. (2003). In vitro studies on the immunoadsorbent for removal of IgA in IgA-nephropathy (II). Chem J Chin Univ-Chin, 24:636–638.
- Chu, J.Q., Zhang, W.H., Yu, Y.T., Zhu, B.R., Chen, C.Z. (2004). In vitro studies of the immunoadsorbent for removal of IgA in IgA-nephropthy (III). Chem J Chin Univ-Chin, 25:1454–1457.