Abstract
This study aims to estimate the effects of using one donor nerve to repair the injured nerve and itself simultaneously by biodegradable chitin conduit. Proximal median nerve served as donor nerve to repair the distal median and whole ulnar nerve. Four months postoperation, the number of myelinated axons and nerve conduction velocities of the distal median and ulnar nerve were (2085 ± 215 and 24.4 ± 5.9 m/s), and (1193 ± 102 and 30.7 ± 11.2 m/s). Recovery of the tetanic muscle forces of the reinvervated muscles were also observed. It suggests that Dor to Dor+Rec neurorrhaphy is a practical method for severe peripheral nerve injury.
INTRODUCTION
Traumatic injury, congenital anomalies, and tumor extirpation may result in damage to or the complete sacrifice of critical nerves. Failure to restore injured nerves can lead to loss of muscle function, impaired sensation, and/or painful neuropathies [Citation1–4]. Traditionally, functional nerve defects have been remedied by many methods, including nerve transfer [Citation5–8], nerve grafts, artificial nerve conduit bridging [Citation9–12], and end-to-side neurorrhaphy [Citation13–20].
However, severe nerve lesions characterized by the absence of a proximal nerve stump are much harder to repair. Because of the long distance for the regenerated axons to go to the distal effectors, the reconstructive effects were very limited, especially for the distal small muscles. As we reported before, peripheral nerves can regenerate more than one shaft in the regenerative distal stump. So, it may be possible to use one nearby nerve or partial axons of a nearby nerve to repair the injured nerve and the donor site simultaneously to shorten the regenerating distance and reduce the donor site morbidity.
Here, we focused on the possibility and reconstruction effects of using a donor nerve to reconstruct the injured site and the donor nerve simultaneously. Results here may give suggestions for the reconstruction of peripheral nerve.
MATERIALS AND METHODS
Animals
We used male New Zealand rabbits that weighed 2.5–3.0 Kg and that were maintained under specific pathogen-free laboratory conditions. The rabbits were separated into 4 groups at random (6 animals in each group). Here, every effort was made to minimize animal suffering and reduce the number of animals used, according to the Chinese guidelines for care and use of laboratory animals.
Surgical Procedures
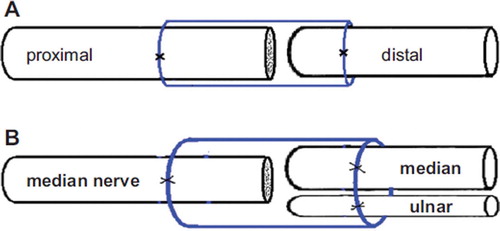
Surgical procedures were carried out under a binocular surgical microscope. Rabbits were anesthetized with sodium pentobarbital (30 mg/kg i.p.). After the anesthesia, rabbit limbs were treated in a sterile manner. An anterior approach was used to expose the median and ulnar nerves at the mid-arm level. In groups 2 and 3, the median/ulnar nerve was transected at the level of 2 cm above elbow, and the proximal nerve segment served as father nerve to repair the distal nerve stump separately (Dor-Dor neurorrhaphy). In group 4, the proximal end of the ulnar nerve was ligated with 10–0 nylon sutures. Then the proximal median nerve served as the donor nerve and was fixed with the distal median and ulnar nerve (Dor-Dor+Rec neurorrhaphy). For this, we used biodegradable chitin conduits (patented by our laboratory and authorized by the state intellectual property office of China, No. ZL01 136314.2; it was an artificial nerve graft consisting of shell chitin that showed satisfactory biocompatibility and degradation characteristics and is now in a preclinical study). 10–0 nylon microsutures were used. The gap between the two nerve segments was kept at 1 mm. The biodegradable chitin conduits were 8 mm long, 0.1 mm thick, with an inner diameter of 4 mm. Subsequently, the muscle incision was sutured and the wound closed using 4–0 nylon sutures ().
Figure 1. Illustration of surgical precedures. A: Proximal median/ulnar nerve segment was served as father nerve to repair the distal nerve stump (Dor-Dor, Group 2, 3); B: Serving as a donor nerve, the proximal median nerve was fixed to the distal stumps of median and ulnar nerve simultaneously, using biodegegradable chitin conduits with a gap of 1 mm. (Dor-Dor+Rec, Group 4).
Tetanic Muscle Force
Tetanic muscle force was measured in the flexor carpi radialis, flexor digitorum superficialis muscle, and flexor carpi ulnaris. Four months postoperatively, the above-mentioned muscles were exposed, and the tendons were cut as distally as possible and ligated. The wrist, the elbow, and the shoulder joints were transfixed by Kirschner wires. The ligature was connected to a force transducer (PCLAB-UE, MicroStar Co. Ltd). The tetanic muscle force was measured at supramaximal stimulation (0.9 mA at 50 Hz) of the reinnervated median and ulnar nerves as previously described [Citation21]. The force was measured on the experimental and the contralateral side and expressed in percentage of the contralateral side.
Electrophysiological Study
Four months postsurgically, before sacrifice of the animals, the repaired median and ulnar nerves were exposed. The stimulating electrodes were placed proximal and distal to the repair site in each group. The recording electrode was placed in the distal reinnervated muscles, while the ground electrode went subcutaneously, between the stimulating and recording electrodes. Rectangular pulses (duration 0.1 ms, 0.9 mA, 10 Hz) were used to stimulate the repaired median and ulnar nerves. Compound muscle action potential was recorded and nerve conduction velocity (NCV; m/s) was obtained semiautomatically by dividing the distance between the two stimulating sites by the difference in the onset latency. The NCV of the contralateral normal median and ulnar nerves was recorded similarly.
Histological Study
The entire median and ulnar nerves were removed en bloc from each rabbit. Tissues were then harvested and fixed in 4% paraformaldehyde in 0.1 M phosphate buffer for 12 h at 4°C. After that, the nerves were rinsed twice in phosphate buffer, and 2 tissue blocks (approx. 5-mm long) were cut, one proximal to the surgical site and one distal to the surgical site. After this step, each sample was postfixed in 1% osmium tetroxide for 6 h, dehydrated through a graded series of ethanol, and embedded in paraffin. Specimen sections were then taken perpendicular to the long axis of the nerve fibers. Myelinated axons were quantified according to the unbiased counting rule. Finally, the total number of myelinated axons was estimated by multiplying the axonal density by the total cross-sectional area of the whole nerve.
Tissue Processing and Staining
Muscles were removed, fixed in 10% buffered formalin, washed in cold water, and transferred to 70% ethanol 24 h later. Following fixation, three representative pieces from the proximal, middle, and distal portions of each muscle were dehydrated in graded ethanol solutions, cleared in xylene, and embedded in paraffin. Five-micrometer sections were mounted on glass slides, deparaffinized in xylene, and rehydrated in graded ethanol solutions. Sections were stained with haematoxylin and eosin. Slides were examined using an Olympus microscope and images were captured.
Statistical Analysis
One-way analysis of variance was employed to compare the number of myelinated nerve fibers, NCV, and tetanic muscle force in all groups. A probability where p< 0.05 was considered significant for all statistical comparisons. All values are presented as the mean ± SD.
RESULTS
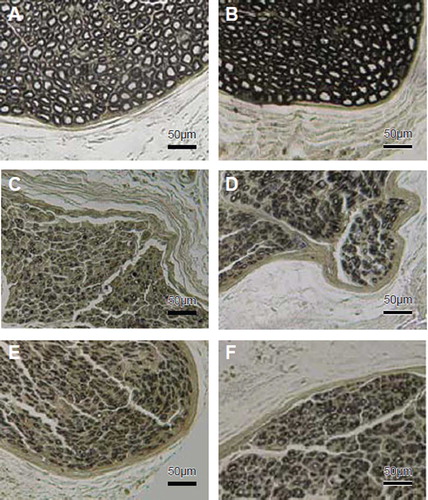
During the 4 months of the follow-up monitoring, no animal died but one rabbit in group 2 and one rabbit in group 4 had a serious infection as a result of unknown causes. The two rabbits were then replaced. After exposure of operation site of the nerve, the biodegradable chitin conduits could not be clearly identified because of the absorption of the conduit and substitution by the fibrous tissues. The nerve stem was continued, which showed that the neonatal neurofibrils have regenerated to the distal part of the injured nerve. However, the regenerated nerves were a little thicker and much dimmer than the normal nerves. The results of quantitative stereological evaluations of the father nerve and the repaired nerve of different groups are summarized in . The single axon count for the sham control group was recorded as the same for the proximal and distal segment, for comparison. The morphologic preservation of all the tissues investigated was good and the myelinated fibers with various calibers were observed in all groups. In some rabbits, in addition to axonal regeneration, there was wallerian-type degeneration, evidenced by myelin springing and break down in the distal part of the ulnar and median nerve. In the “Dor-Dor+Rec neurorrhaphy” group, the regenerated axons can be observed in both median and ulnar nerves. There was no significant morphologic difference of the regenerated axons among different experimental groups ().
Table 1. Comparison of myelinated axon numbers for all groups.
Figure 2. Histological sections through the reconstructed nerves 4 months after surgery. Proximal part of the median nerve (A) and ulnar nerve (B). Distal stump of transected median (C, Dor-Dor, Group 2) or ulnar (D, Dor-Dor Group 3) nerve neurorrhaphy; Distal median (E) and ulnar (F) stump of the group (Dor-Dor+Rec Group 4) in which whole proximal median proximal median nerve was served as donor nerve to reconstruct the distal median and ulnar nerve stump.
Histomorphometrical evaluations revealed that the axon number of the distal segment of the median and ulnar nerve in the control group (group 1) are 2343 ± 304 and 1633 ± 223 respectively,which represent the axon number of the normal median and ulnar nerves. These values are 2225 ± 369 and 1432 ± 311 in the groups of transected median and ulnar nerve neurorrhaphy (group 2 and 3). So, the ratios of the regenerated axon number to the donor axon number (amplifying ratio as described before) in these two groups were 0.91 and 0.88, respectively, with an obvious ratio of distal acceptor axon number to the proximal donor axon number (RDP) of about 1. The axon numbers are 2085 ± 215 and 1193 ± 102 in the “Dor-Dor+Rec neurorrhaphy” group with the RDP value of 1.69 and amplifying ration of 1.39, respectively ().
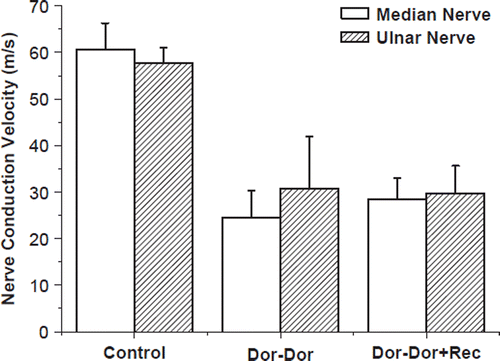
At 4 months postoperatively, electrophysiological assessment was conducted prior to the sacrifice of the animals. The motor nerve conduction velocity of the normal median and ulnar nerve was 60.6 ± 5.7 and 57.7 ± 3.5 m/s, respectively. In the groups of transected median and ulnar nerve neurorrhaphy (group 2 and 3), these values are 28.4 ± 4.6 and 29.8 ± 5.8 m/s separately. The values are 24.4 ± 5.9 and 30.7 ± 11.2 m/s in the “Dor-Dor+Rec neurorrhaphy” group ().
Figure 3. Nerve conduction velocities of rabbit regenerative median and ulnar nerve. Data were collected 4 months after reconstructive surgery. Electrophysiological recording was performed on the distal part of the median or ulnar nerve. There is no significant difference among the transected median/ulnar nerve neurorrhaphy groups (Dor-Dor, group 2, 3) and the group (Dor-Dor+Rec, group 4) in which proximal median nerve served as donor nerve to reconstruct the distal median and ulnar nerve stump.
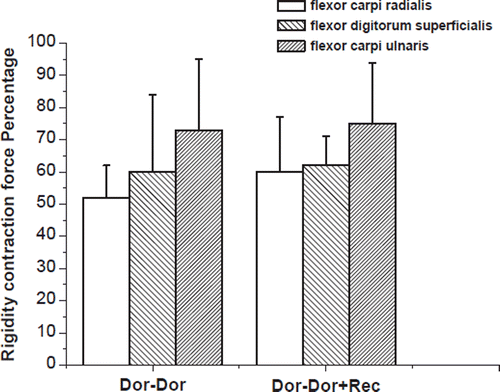
Electrical stimulation of the regenerated median or ulnar nerves resulted in muscle contraction of the forearm muscles innervated by the attached nerves (wrist/toe extension and wrist/toe flexion, respectively). Measurement of the tetanic muscle force in the flexor carpi radialis muscle, flexor digitorum superficialis and the flexor carpi ulnaris, respectively, showed that the ipsilateral side had values of 60 ± 17%, 62 ± 9%, 75 ± 19% of the contralateral side, after the distal ulnar and median nerves were repaired by their proximal stem. The values were 52 ± 10%, 60 ± 24%, 73 ± 22% in the “Dor-Dor+Rec neurorrhaphy” group ().
Figure 4. The tetanic muscle force in the flexor carpi radialis muscle, flexor digitorum superficialis and the flexor carpi ulnaris. It showed that the ipsilateral side had values of 60 ± 17%, 62 ± 9%, 75 ± 19% of the contralateral side, after the distal ulnar and median nerves were repaired by their own proximal stem (Dor-Dor, Group 2, 3). And the values were 52 ± 10%, 60 ± 24%, 73 ± 22% in the group where distal ulnar and median nerves were reconstructed by the whole proximal median nerve (Dor-Dor+Rec, Group 4).
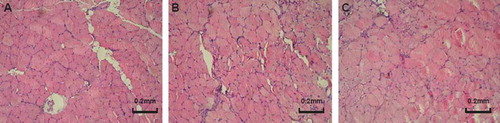
Light microscopy analysis showed that the normal muscles had the regular aspect of muscle fibers, while the reinnervated muscles were substantially different from that of normal muscle. The changes include variation in muscle fiber size, degeneration and incomplete regeneration of fibers, proliferation of connective tissue, and infiltration of the damaged muscle with immune response cells. However, there seems no significant difference among the experimental groups with different reconstructing protocols ().
Figure 5. Photomicrographies of the flexor carpi ulnaris (FCU) histological analysis (HE staining). (A) FCU muscle of the control group showing the regular aspect of muscle fibers; (B) FCU muscle of the transected ulnar nerve neurorrhaphy group (Dor-Dor, Group 3); (C) and group in which whole proximal median nerve was served as donor nerve to reconstruct the distal median and ulnar nerve stump (Dor-Dor+Rec, Group 4).
DISCUSSION
In this research, we focused on the possibility of using a donor nerve to reconstruct the injured nerve and the donor site simultaneously to reduce the loss of donor site function and the influence of the donor nerve axon number to the reconstruction effect of the injured nerve.
The biodegradable conduit used here was made from shell chitin with partial de-acetyl procedure. Animal experiments showed that such a conduit could retain its structure for at least 6 weeks in vivo to support axons regeneration, and be absorbed completely within 3 months. It also showed good biologic compatibility with Schwann cells and neurons, and the degradation residue had no toxicity to the imbedding body. It has been patented by our laboratory and authorized by the state intellectual property office of China (No. ZL01 136314.2). This product is under a preclinical study. Our previous study also showed that the suitable regeneration gap for rat peripheral nerve was 1– 2 mm, and it could not facilitate the nerve regeneration effects when the gap exceeded 5 mm [Citation23]. In this study, the gap between the proximal and distal segments was 1 mm, and the specimens were harvested 4 months after the operation. According to our previous results, with such a gap and the time period, the conduit could support axons regenerating from the donor side to the distal nerve stump with the most advantage.
The most important finding of this study is that it is possible to use one nerve to repair the injured nerve and donor site simultaneously (Dor to Dor+Rec neurorrhaphy). Here, the median nerve served as the father nerve to restore the distal median and ulnar nerve simultaneously. Both recovery of the donor site and injured nerve were observed in the experiment. There seemed to be no significant difference in the reconstructed function of distal regenerated median and ulnar nerve between the Dor to Dor+Rec neurorrhaphy (group 4) and the Dor to Dor neurorrhaphy (group 2, 3). As we reported before, peripheral nerve neuron can regenerate and remain more than one shaft in the regenerative distal stump. Half to one-third of the axons of the acceptor nerve may be considered as a suitable number of donor nerve axons [Citation22]. Here, the ratio of proximal donor axon number to the distal acceptor nerve axon number is about 1:1.69 in group 4. The ratio of the proximal donor axon number to the distal acceptor nerve axon number of the present experiment is within the ratio limit for the acceptable reconstructive effects. So, with this kind of reconstruction method (Dor to Dor+Rec neurorrhaphy) the regenerated axons can regenerate into both the donor site and the injured site. That means separating a nearby intact nerve as donor nerve to repair the injured nerve and donor site simultaneously can not only get good reconstructive effects from the injured nerve but also restore most function of the donor site.
The time for the reconstruction of an injured nerve is another important factor that can greatly affect the reconstructive effects. The reconstructive time is determined by the distance from the injured nerve site to its effectors. So, the longer the distance, the worse reconstructive effect could be obtained. Thus, if the injured location is too far away from its effectors, no good prognosis could be obtained, even if the best repairing technology is used. It is because the denervated effectors, such as small muscles, have degenerated to an unrecoverable level before the nerve reinnervation. Based on the results of this experiment, the injured never and donor site could be repaired by the same nearby donor nerve or even partial nerve axons. We can choose the operation site as closely to the effectors as we can to reduce the reinnervating time. Therefore, both the donor site and the injured nerve effectors can be reinnervated quickly, and thus the reconstructive effects were enhanced.
What is the indication for “Dor to Dor+Rec neurorrhaphy”? In common peripheral nerve injury, the continuity is reestablished between proximal and distal nerve stumps by end-to-end (terminoterminal) suture. However, severe nerve lesions (e.g. root avulsion of brachial plexus) characterized by the absence of a proximal nerve stump are much harder to repair. Traditionally, the healthy C7 nerve root of the opposite side upper extremity was isolated and an autografting nerve was used as the bridge between the C7 root and the distal stem to restore the injured nerve. Because of the long distance for the regenerated axons to go to the distal effectors, the reconstructive effects were very limited. As described here, using a nearby nerve to repair the injured nerve and the donor site simultaneously can greatly shorten the regenerating distance. The denervated effectors can be reinnervated in the fastest manner. Although there seems to be an unavoidable injury to the nearby healthy nerve, if both the recovery of the donor site is within an acceptable level and the injured fields are restored too, Dor to Dor+Rec neurorrhaphy may play a great role in such nerve injuries.
However, the assessment of the function recovery in this experiment was based on the histological and electrophysiological methods. Although the slices showed the successful regeneration and electrical stimulation responded with contraction of the reinnervated muscles, it could only be a proof of structural reinnervation. Axonal pruning is commonly considered to be at the basis of the functional adaptation to the new connections. The functional recovery needs not only the connection between the peripheral nerve and its effectors, but also the effective control from the central nerve system on the peripheral reinnervated effectors. It is obvious that when a donor nerve was used to repair the donor site and the injured nerve simultaneously, the original father nerve would control not only the effectors innervated, but also control other effectors after reconstruction. Sometimes these effectors may have opposite functions like antagonistic muscles in the locomotor system. So, brain reorganization related to major changes in the peripheral connections is a more important issue for the real function recovery of the reconstructed nerves. What happens to the central neuronal circuitries? What are the brain plasticity mechanisms for adapting to environmental perturbations? Evidence from the present experiment could not give a good answer to these questions.
ACKNOWLEDGMENTS
This work was supported by a grant from Chinese National 863 Project (No.2002AA205071), National Natural Science Fund of China (No. 30271306), New Teacher Fund (No. 801985) and the Chinese National Natural Science Fund for the Outstanding Youth (No. 30625036).
Declaration of interest: This work was supported by Chinese National Natural Science Fund for Outstanding Youth (30625036), Chinese 973 Project Planning (2005CB522604), Chinese National Natural Science Youth Fund (30801169), Chinese National Natural Science Fund (30971526), Chinese New Teacher Fund (200800011102).
REFERENCES
- Dahlin, L.B., Lundborg, G. (2001). Use of tubes in peripheral nerve repair. Neurosurg Clin N Am 12(2):341–52.
- Frostick, S.P., Yin, Q., Kemp, G.J. (1998). Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery 18(7):397–405.
- Lundborg, G., Dahlin, L., Danielsen, N., Zhao, Q. (1994). Trophism, tropism, and specificity in nerve regeneration. J Reconstr Microsurg 10(5):345–54.
- Seckel, B.R. (1990). Enhancement of peripheral nerve regeneration. Muscle Nerve 13(9):785–800.
- Chuang, D.C. (1995). Neurotization procedures for brachial plexus injuries. Hand Clin 11(4):633–45.
- Oberlin, C., Beal, D., Leechavengvongs, S., Salon, A., Dauge, M.C., Sarcy, J.J. (1994). Nerve transfer to biceps muscle using a part of ulnar nerve for C5-C6 avulsion of the brachial plexus: anatomical study and report of four cases. J Hand Surg Am 19(2):232–7.
- Sungpet, A., Suphachatwong, C., Kawinwonggowit, V., Patradul, A. (2000). Transfer of a single fascicle from the ulnar nerve to the biceps muscle after avulsions of upper roots of the brachial plexus. J Hand Surg Br 25(4):325–8.
- Tung, T.H., Mackinnon, S.E. (2001). Flexor digitorum superficialis nerve transfer to restore pronation: two case reports and anatomic study. J Hand Surg Am 26(6):1065–72.
- Evans, G.R., Brandt, K., Widmer, M.S., Lu, L., Meszlenyib, R.K., Gupta, P. K, . (1999). In vivo evaluation of poly (L-lactic acid) porous conduits for peripheral nerve regeneration. Biomaterials 20(12):1109–15.
- Madihally, S.V., Matthew, H.W. (1999). Porous chitosan scaffolds for tissue engineering. Biomaterials 20(12): 1133–42.
- Matsumoto, K., Ohnishi, K., Kiyotani, T., Takashi, S., Ueda, H., Nakamura, T., . (2000). Peripheral nerve regeneration across an 80-mm gap bridged by a polyglycolic acid (PGA)-collagen tube filled with laminin-coated collagen fibers: a histological and electrophysiological evaluation of regenerated nerves. Brain Res 868(2):315–28.
- Vasconcelos, B.C., Gay-Escoda, C. (2000). Facial nerve repair with expanded polytetrafluoroethylene and collagen conduits: an experimental study in the rabbit. J Oral Maxillofac Surg 58(11):1257–62.
- Kostakoglu, N. Motor and sensory reinnervation in the hand after an end-to-side median to ulnar nerve coaptation in the forearm. Br J Plast Surg 52(5):404–7.
- Matsumoto, K., Ohnishi, K., Sekine, T. (2000). Use of a newly developed artificial nerve conduit to assist peripheral nerve regeneration across a long gap in dogs. Asaio J 46(4):415–20.
- McCallister, W.V., Tang, P., Trumble, T.E. (1999). Is end-to-side neurorrhaphy effective? A study of axonal sprouting stimulated from intact nerves. J Reconstr Microsurg 15(8):597–603; discussion 603–4.
- Okajima, S., Terzis, J.K. (2000). Ultrastructure of early axonal regeneration in an end-to-side neurorrhaphy model. J Reconstr Microsurg 16(4):313–23; discussion 323–6.
- Shah, M.H., Kasabian, A.K., Karp, N.S. (1997). Axonal regeneration through an autogenous nerve bypass: an experimental study in the rat. Ann Plast Surg 38(4):408–14; discussion 414–5.
- Viterbo, F., Trindade, J.C., Hoshino, K., Mazzoni, A. (1994). Two end-to-side neurorrhaphies and nerve graft with removal of the epineural sheath: experimental study in rats. Br J Plast Surg 47(2):75–80.
- Yoleri, L., Songur, E., Yoleri, O. (2000). Reanimation of early facial paralysis with hypoglossal/facial end-to-side neurorrhaphy: a new approach. J Reconstr Microsurg 16(5):347–55; discussion 355–6.
- Zhao, J.Z., Chen, Z.W., Chen, T.Y. (1997). Nerve regeneration after terminolateral neurorrhaphy: experimental study in rats. J Reconstr Microsurg 13(1):31–7.
- Scherman, P., Lundborg, G., Kanje, M., Dahlin, L.B. (2001). Neural regeneration along longitudinal polyglactin sutures across short and extended defects in the rat sciatic nerve. J Neurosurg 95(2):316–23.
- Jiang, B.G., Yin, X.F., Zhang, D.Y., Fu, Z.G., Zhang, H.B. (2007). Maximum number of collaterals developed by one axon during peripheral nerve regeneration and the influence of that number on reinnervation effects. Eur Neurol 58(1):12–20.
- Jiang, B.L., Zhang, P.X., Jiang, B.G. (2010). Advances in small gap sleeve bridging peripheral nerve injury. Artif Cells Blood Substit Immobil Biotechnol. 38(1):1–4.