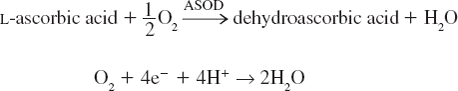Abstract
Abstract: Ascorbate oxidase purified from Lagenaria siceraria fruit was immobilized onto epoxy resin “Araldite” membrane with 79.4% retention of initial activity of free enzyme. The biosensor showed optimum response within 15s at pH 5.8 and 35°C, which was directly proportional to ascorbate concentration ranging from 1-100μM. There was a good correlation (R2 = 0.99) between serum ascorbic acid values by standard enzymic colorimetric method and the present method. The enzyme electrode was used for 200 times without considerable loss of activity during the span of 90 days when stored at 4°C.
INTRODUCTION
Ascorbic acid is an important analyte that presents in many biological fluids, juices, soft drinks, pharmaceutical formulations, etc., and many analytical aspects related to this analyte have attracted a great deal of attention over the years [Citation1,Citation2]. It is used for therapeutic purpose and in biological processes like production of collagen and nor-epinephrine [Citation3]. Ascorbic acid has immense importance in biological systems, as it potentiates nitric oxide synthesis in endothelial cells and plays an important role in a number of metabolic functions, including activation of vitamin B, folic acid, the conversion of cholesterol to bile acids, and the conversion of amino acid (tryptophan) to the neurotransmitter (serotonin). As an excellent source of electrons, ascorbic acid donates electrons to free radicals such as hydroxyl and superoxide radicals and quenches their reactivity [Citation4]. Dehydroascorbate (oxidized form) also causes stimulation of pentose phosphate pathway and glutathione levels [Citation5]. The recent advances in the foodstuffs and pharmaceutical industries and requirement for nutritional assessments necessitate the development of a selective, simple, and accurate method for determination of ascorbic acid. Among the various methods available for determination of ascorbic acid such as colorimetric (DCPIP titration) method [Citation6], enzymic colorimetric [Citation7], UV spectrophotometric method [Citation8], high performance liquid chromatography (HPLC) [Citation9], amperometric biosensors based on immobilized ascorbate oxidase are most simple, rapid, sensitive, and specific and can differentiate between the L and D form of ascorbate. In order to construct ascorbate biosensor, ascorbate oxidase has been immobilized on CH-Sepharose via carbodiimide [Citation10], plant tissue- membrane [Citation11], alkylamine glass beads [Citation12], double electropolymer modified Pt electrode [Citation13], ethylene–vinylacetate membrane [Citation14], teflon membrane for dissolved O2 measurement [Citation15], carbon paste modified electrode [Citation16], β-cyclodextrin–ferrocene inclusion complex modified carbon paste electrode [Citation17], glassy carbon [Citation18], antibodies packed in a glass capillary column for organophosphorous pesticides monitoring [Citation19], polystyrene membranes [Citation20], ferrocene-L-cysteine self-assembled supramolecular film modified electrode [Citation21], nylon net membrane [Citation22], electrochemically etched platinum microelectrode [Citation23], multilayer films of carbon nanotubes and redox polymer on screen-printed carbon electrodes [Citation24], micelle membrane coated on aminated glassy carbon electrode and gold electrode [Citation25], Cu(II) zeolite-modified electrode [Citation26], and dopamine poly(acriflavine)-modified electrode [Citation27].
Recently, we have reported an epoxy resin “Araldite” (brand name) membrane as a support for immobilization of uricase to construct a dissolved oxygen (DO) metric uric acid biosensor [Citation28]. The membrane is composed of epoxy resins having epoxy groups on both sides of single epoxy resin, forming a highly crosslinked network, which make it long-lived and water-permeable [Citation28]. The present report describes the construction of an amperometric ascorbate biosensor employing an epoxy resin (Araldite) membrane bound ascorbate oxidase purified from green fruits of Lagenaria siceraria.
MATERIALS AND METHODS
Materials
Sephadex G-100 and DEAE—Sephacel were from Sigma Aldrich (USA). Serum samples of normal/healthy persons were collected at local Pt BDS Postgraduate Institute of Medical Sciences (PGIMS) hospital. All other chemicals were of AR grade. Epoxy resin and polyamine crosslinker marketed under the brand name “Araldite” manufactured by Huntsman Advanced Materials Pvt. Ltd., Mumbai, and fruits (lemon, apple, grapefruit, and orange) were purchased from a local market.
Collection of Experimental Fruits
Fresh green bottle gourd (Lagenaria siceraria) fruits of 10–15 cm diameter were collected from a nearby village during the months of June-July (30±5°C) and brought to the laboratory in a ice bath, washed in distilled water, and stored at 4°C until use.
Extraction and Purification of Ascorbate Oxidase
Ascorbate oxidase was extracted and purified from fresh green bottle gourd (Lagenaria siceraria) fruits using a combination of 65% ammonium sulphate precipitation, gel filtration on Sephadex G-100, and ion-exchange chromatography on DEAE-Sephacel as described [Citation29]. The purified enzyme exhibited a single band in simple polyacrylamide gel electrophoresis (PAGE) using coomassie blue as protein stain, indicating its apparent homogeneity. The purified enzyme had an activity 9.6 unit/ml.
Assay of Ascorbate Oxidase
The assay of ascorbate oxidase was carried out as described by Oberbacher and Vines [Citation8] with slight modification. The reaction mixture contained 2.9 ml phosphate/EDTA buffer (0.1 M, pH-5.6), 0.1 ml L-ascorbic acid (0.005 M), and 0.1 ml (0.96 U) enzyme. The blank contained 3.0 ml of 0.1 M phosphate/EDTA buffer pH 5.6 and 0.1 ml ascorbic acid (0.005 M) A265 was read in a UV and visible spectrophotometer (Make: Shimadzu 1700, Japan). The activity of enzyme was calculated as follows:
[e=13.386 (extinction coefficient of dehydroascorbate); total volume =3.1; enzyme volume=0.1 ml]
Preparation of Ascorbate Oxidase–epoxy Resin Biocomposite Membrane
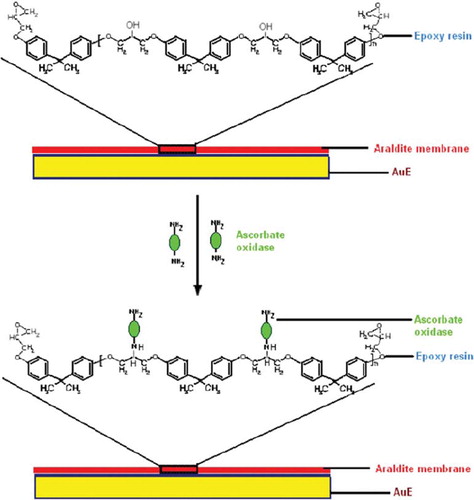
It was prepared as described [Citation28]. The epoxy resin and hardener of “Araldite” were mixed on a plastic (polythene) piece (size 4 cm ×4 cm) in 85:15 ratio at room temperature for 5 min. Purified enzyme (2 ml, protein content 50μg/ml) was added to this mixture and spread equally to polymerize and allowed to cross-link for 48 h. “Araldite” membrane with entrapped ascorbate oxidase was stripped off the plastic piece and washed with 0.1 M phosphate/EDTA buffer, pH 5.6.
Scanning Electron Microscopy (SEM) of Araldite Membrane
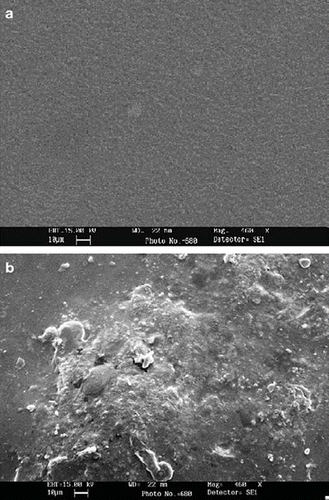
Scanning electron microscopy (SEM) of “Araldite” membrane alone and its conjugate with ascorbate oxidase was carried out at Sophisticated Analytical Instrumentation Facility (DST), All India Institute of Medical Sciences (AIIMS), New Delhi, to confirm the immobilization.
Construction of Amperometric Ascorbate Biosensor and its Response Measurement
An amperometric ascorbate biosensor was constructed by mounting ascorbate oxidase-epoxy resin membrane biocomposite onto Au electrode (1.5 ×0.05 cm) with a parafilm and connecting this working electrode along with a silver/silver chloride (Ag/AgCl) reference electrode and Cu as auxiliary electrode to a three terminal electrometer (Keithley, 6215A/E Japan). To test the activity of the three electrode system, it was immersed into a mixture of 2.9 ml 0.1 M phosphate-EDTA buffer, pH 5.6 and 0.1 ml ascorbic acid (0.005 M). The electrode was polarized by applying different potential in the range 0.1 to 0.8 V. The potential was noted at which the maximum current was generated. The following electrochemical reactions occur during measurement:
*ASOD=ascorbate oxidase.
Optimization of Ascorbate Biosensor
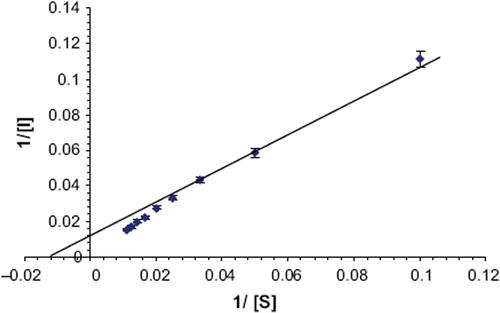
The optimum working conditions of biosensor/kinetic properties of immobilized ascorbate oxidase were studied at 0.6 V (voltage at which maximum current was generated) and compared with those of free enzyme. To determine the optimum pH, the pH of reaction buffer was varied from pH 3.0 to 6.5 using the following buffer, each at a final conc. of 0.1 M: pH 3.0 to 5.0 sodium citrate and pH 5.5 to 6.5 phosphate-EDTA buffers. Similarly, the optimum temperature was studied by incubating reaction mixture at different temperatures ranging from 15 to 45°C at an interval of 5°C in a controlled temperature water bath. To study the effect of substrate concentration, the ascorbic acid concentration was varied from 1 to 100 μM. Km and Imax were calculated from Lineweaver–Burk (LB) plot.
Amperometric Determination of Ascorbic Acid in Serum and Fruit
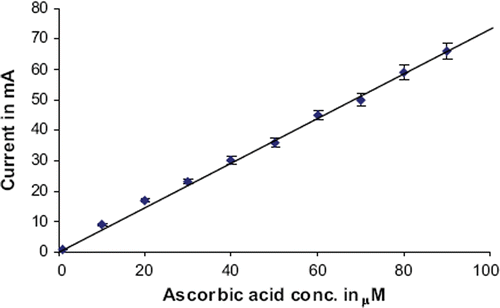
Fresh serum samples (0.5 ml) from apparently healthy persons were collected at local Pt BDS Postgraduate Institute of Medical Science Hospital, Rohtak. To prepare the fruit sample, the average-sized fruits (lemon, orange, grapefruit, and apple) were peeled off and their juices were collected separately by pressing them. The juice was centrifuged at 15000 ×g for 15 min and supernatant was collected. The biological fluids were analyzed as described for response measurement of biosensor, except that the ascorbic acid solution was replaced by a biological sample. The ascorbate content in biological fluid was extrapolated from standard curve between ascorbate conc. vs current in mAmp ().
Effect of Serum Substances
To study the effect of important serum metabolites in the present method, 0.1 ml aqueous solution of the following compounds was added to the reaction mixture, each at a final conc. of 1 × 1024M: glucose, fructose, cysteine, sucrose, urea, cholesterol, and uric acid.
Reuse of Immobilized Ascorbate
To reuse the enzyme electrode, it was washed 3–4 times with the reaction buffer (0.1 M phosphate/EDTA buffer pH 5.6), and dried in between folds of tissue paper before its use in next assay. The enzyme electrode was stored in the same buffer at 4°C when not in use.
RESULTS AND DISCUSSION
Immobilization of Lagenaria siceraria Fruit Ascorbate Oxidase onto “Araldite” Membrane
An ascorbate oxidase purified from fresh green bottle gourd (Lagenaria siceraria) fruit was immobilized covalently onto “Araldite” membrane with 79.4% retention of initial activity of free enzyme. The immobilization of enzyme was confirmed by scanning electron microscopic (SEM) study of epoxy resin membrane before and after immobilization of ascorbate oxidase. Araldite membrane with immobilized enzyme had clusters along some beaded structures, which were not observed in the membrane alone ( & ). These clusters might be due to both adsorption and chemical coupling of enzyme onto the membrane. The –OH groups of epoxy (based on dihydroxydiphenylpropane and epichlorohydrin) containing polymers react with bifunctional polyamine and –NH2 groups of enzyme to form a -C-N- linked enzyme–epoxy amine resin composites [Citation28] ().
Optimization of Ascorbic Acid Biosensor
There were minor changes in kinetic properties of enzyme after immobilization (). The optimal pH of biosensor/immobilized ascorbate oxidase was 5.8, which is slightly higher than that of the free enzyme (pH 5.5) [Citation30]. However, immobilized ascorbate oxidase was much more stable in the acidic pH range than the alkaline range. This change in optimum pH of enzyme could be due to the repulsive forces between the hydroxyl groups of the epoxy resin polymer and hydronium ions [Citation23–26]. The optimum temp of biosensor/immobilized ascorbate oxidase was 35°C, which is lower than that of free enzyme (40°C) [Citation30]. The decrease in the optimum temperature might be due to the improvement in the enzyme rigidity upon immobilization through covalent binding. Similar displacement of optimum temperature for immobilized enzymes has been reported earlier, but the extent of displacement differed from matrix to matrix and with the kind of interaction between the enzyme and matrix [Citation31,Citation32]. The rate of reaction of immobilized ascorbate oxidase was linear up to 15 s, after which it was constant. There was a hyperbolic relationship between biosensor response/immobilized ascorbate oxidase activity and ascorbic acid concentration up to 100 μM, after which it was constant. The L B plot () for immobilized ascorbate oxidase gave a Km value of 83.3 μM for ascorbate, which is higher than that of free enzyme, revealing decreased affinity of enzyme towards its substrate after immobilization; Imax for sensor was 80.3 mA/min. summarizes comparison of the present ascorbate biosensor with earlier biosensors. An amperometric method for determination of ascorbic acid in biological materials was developed using the present biosensor. The biosensor has the advantage that it employs covalently immobilized enzyme, which had better biomolecule activity, reduced nonspecific adsorption, and greater stability [Citation33]. The following criteria were studied to evaluate the method.
Table 1. A comparison of ascorbate biosensors using plant ascorbate oxidase
Linearity
There was a linear relationship between current (mA) and ascorbic acid concentration ranging from 1 to 100 μM in reaction mixture, which is comparable to earlier reported biosensors (10 to 600 μM) [Citation25,Citation34,Citation35] ().
Limit of Detection
Limit of detection of the present method was 1.0 μM, which is better/lower than earlier biosensors (2μM - 4μM) [Citation25,Citation35] ().
Recovery
The analytic recovery of added ascorbate into serum (1.0 mg/dl and 2.0 mg/dl final concentration in reaction mixture) was 85.9±3.1% and 91.5±3.4%, respectively (). These results are comparable to earlier reported o-phenylenediamine mediated ascorbate oxidase assay (>95%) [Citation7] and biosensor based on carbon paste modified (98.1 to 102.1%) [Citation35].
Table 2. Analytical recovery of added ascorbate in serum, as measured by ascorbate biosensor using epoxy resin membrane bound bottle gourd fruit ascorbate oxidase
Precision
To study the reproducibility and reliability of the present method, the ascorbate content in six serum samples was determined six times on a single day (within batch) and again after storage at −20°C for one week (between batch). The results showed that determinations were consistent and within and between batch coefficients of variation (CVs) were <8.19% and <10.4%, respectively (), which indicate the reliability and consistency of the present method. The present CVs are comparable to earlier CVs by colorimetric method, HPLC (<5.0% within) [Citation9], and also by o-phenylenediamine mediated ascorbate oxidase assay (<4.3% within and <6.7% between) [Citation7].
Table 3. Ascorbate level in sera of apparently healthy adults, as determined by ascorbate biosensor based on epoxy resin membrane bound bottle gourd fruit ascorbate oxidase
Accuracy
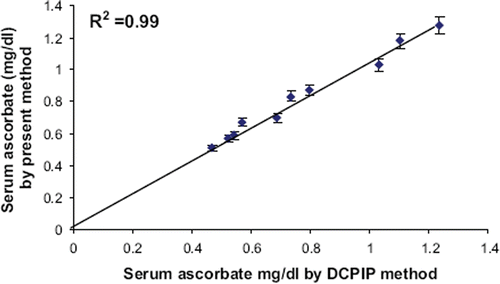
To determine the accuracy of the method, the level of ascorbate in 10 serum samples was determined by standard DCPIP titrimetric method (x) and by the present method (y). The serum ascorbate values obtained by both the methods matched with each other with a good correlation (R2 = 0.99, significant at 0.1% level) (). Evaluation studies showed that the method was fairly reliable with high recovery and in agreement with the standard method.
Serum Ascorbate Determination
The serum ascorbate level in apparently healthy adults as measured by present biosensor, ranged from 29.0 μM to 86.8 μM with a mean of 70.6 μM (), which is in the normal established range (22.7 μM – 85.2 μM).
Table 4. Determination of ascorbic acid in various fruit juices by amperometric ascorbate biosensor based on epoxy resin membrane bound bottle gourd fruit ascorbate oxidase
Determination of Ascorbate in Fruits
The content of ascorbate in various fruits was determined by the present biosensor and the results are presented in . These values are in good agreement with earlier reports [Citation25,Citation36].
Table 5. Effect of various serum substances on response of ascorbate biosensor based on epoxy resin membrane bound bottle gourd fruit ascorbate oxidase
Interference Study
Among the various serum substances tested, such as glucose, fructose, urea, cysteine, sucrose, urea, cholesterol and uric acid, none caused significant interference in biosensor response (Table 6).
Reusability and Storage
The enzyme electrode was reused for 200 times during the span of 90 days when stored in 0.1 M sodium phosphate EDTA buffer pH 5.6 at 4°C. This reusability and stability is higher than those of earlier biosensors [Citation15].
CONCLUSIONS
An epoxy resin membrane ascorbate oxidase bioconjugate was employed successfully to construct an amperometric ascorbate biosensor. An epoxy resin membrane based electrode revealed improved analytical performance in terms of sensitivity, i.e. minimum detection limit (1.0μM), high recovery (85.9% and 91.5%), precision (within and between batch CVs <8.19% & <10.4%), reproducibility, stability up to 3 months, and no interference by many serum co-substances. The sensing element is likely to be useful for a miniaturized biosensor.
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Arya, S.P., Mahajan M., Jain, P. (1998). Photometric Methods for the Determination of Vitamin C. Anal. Sci. 14: 889–896.
- Arya, S.P., Mahajan M., Jain, P. (2000). Non-spectrophotometric methods for the determination of. Vitamin C. Anal. Chim. Acta 417: 1–14.
- Heller, R., Münscher-Paulig, F., Gräbner R., Till, U.J. (1999). L-Ascorbic Acid Potentiates Nitric Oxide Synthesis in Endothelial Cells. Biol. Chem. 274: 8254–8260.
- Bendich, A. (1990). Antioxidant micronutrients and immune responses. A. Bendich, R.K. Chandra. Micronutrients and immune functions. N.Y. Academy of Sciences, New York, 175.
- Puskas, F., Gergely P., Perl, A. (2000). Stimulation of the pentose phosphate pathway and glutathione levels by dehydroascorbate. J FASEB 14: 1352–61.
- Roe, J.H. (1954). Determination of ascorbic acid by titration method using DCPIP. Methods Biochem. Anal. 1: 115–118.
- Ihara, H., Shino, Y., Aoki, Y., Hashizume N., Minegishi N. (2000). A simple and rapid method for the routine assay of total ascorbic acid in serum and plasma using ascorbate oxidase and o-phenylenediamine. J. Nutr. Sci. Vit. 46: 321–4.
- Oberbacher M.F., Vines, H.M. (1963). Assay procedure for ascorbate oxidase by UV spectrophotometer. Nature 197: 1203–1204.
- Lykkesfeldt, J., Loft S., Poulsen, H.E. (1995). Determination of ascorbic acid and dehydroascorbic acid in plasma by HPLC with colorimetric detection. Anal. Biochem. 229: 329–335.
- Stevanato, R., Avigliano, L., Finazzi-Agro A., Rigo, A. (1985). Determination of ascorbic acid with immobilized green zucchini ascorbate oxidase. Anal. Biochem. 149: 537–42.
- Macholan L., Chmelíkova, B. (1986). Plant tissue-based membrane biosensor for L-ascorbic acid. Anal. Chim. Acta. 185: 187–193.
- Marques E.T., Lima-Filho, J.L. (1992). Ascorbic acid biosensor using ascorbate oxidase immobilized on alkylamine glass beads. Appl. Biochem. Biotechnol. 32: 73–78.
- Losito I., Zambonin, C.G. (1996). Double electropolymer modified platinum electrode to follow the kinetic process H2O2 + ascorbic acid. Influence of the reaction on amperometric biosensor applications. J. Electroanal. Chem. 410: 181–187.
- Fernandes, J.C.B., Kubota L.T., Neto, G.D.O. (1999). Potentiometric biosensor for l-ascorbic acid based on ascorbate oxidase of natural source immobilized on ethylene-vinylacetate membrane. Anal. Chim. Acta. 385: 3–12.
- Akyilmaz E., Dinçkaya, E. (1999). A new enzyme electrode based on ascorbate oxidase immobilized in gelatin for specific determination of L-ascorbic acid. Talanta, 50: 87–93.
- Fatibello-Filho O., Vieira, I.C. (2000). L-ascorbic acid determination in pharmaceutical formulations using a biosensor based on carbon paste modified with crude extract of zucchini (Cucurbita pepo). J. Braz. Chem. Soc. 11: 412–418.
- Guorong, Z., Xiaolei, W., Xingwang S., Tianling, S. (2000). β-Cyclodextrin–ferrocene inclusion complex modified carbon paste electrode for amperometric determination of ascorbic acid. Talanta, 51: 1019–1025.
- Khorasai-Motlagh, M., Noroozifar, M. (2004). Electrocatalytical determination of ascorbic acid using glassy carbon modified with nickel (II) macro cycle containing dianionic tetraazaannulene ligand. Turk. J. Chem. 28: 369–378.
- Rekha, K., Gouda, M.D., Thakur M.S., Karanth, N.G. (2000). Ascorbate oxidase based amperometric biosensor for organophosphorous pesticide monitoring. Biosens. Bioelectron. 15: 499–502.
- Wang X., Uchiyama, S. (2006). Roles of chemical gas sensors in the field of safety. Chem. Sens. 22: 22–24.
- Wang S., Du, D. (2004). Differential pulse voltammetry determination of ascorbic acid with ferrocene-L-cysteine self-assembled supramolecular film modified electrode. Sens. Actuat. 97: 373–378.
- Tomita, I.N., Manzoli, A., Fertonani F.L., Yamanaka, H. (2005). Amperometric biosensor for ascorbic acid. Eclet. Quím. 30: 37–42.
- Paixao, T.R.L.C., Lowinsohn D., Bertotti, M. (2006). Use of an Electrochemically Etched Platinum Microelectrode for Ascorbic Acid Mapping in Oranges. J. Agric. Food Chem. 54: 3072–3077.
- Sha, Y., Qian, L., Ma, Y., Bai H., Yang, X. (2006). Multilayer films of carbon nanotubes and redox polymer on screen-printed carbon electrodes for electrocatalysis of ascorbic acid. Talanta, 70: 556–560.
- Wang, X., Watanabe H., Uchiyam, S. (2008). Amperometric l-ascorbic acid biosensors equipped with enzyme micelle membrane. Talanta, 74: 1681–1685.
- Tahereh R., Taher, M. A. (2009). A new method for electrocatalytic oxidation of ascorbic acid at the Cu(II) zeolite-modified electrode. Talanta, 78: 743–747.
- Niena, P.C., Chena P.Y., Chuan Ho, K., (2009). On the amperometric detection and electrocatalytic analysis of ascorbic acid and dopamine using a poly(acriflavine)-modified electrode. Sens. Actuat. 140: 58–64.
- Arora, J., Nandwani, S., Bhambi M., Pundir, C.S. (2009). Fabrication of dissolved O2 metric uric acid biosensor using uricase epoxy resin biocomposite membrane. Anal. Chim. Acta. 647: 195–201.
- Lucas, K., Boland, M.J., Schubert, K.R. (1983). Uricase from soybean root nodules: purification, properties and comparison with the enzyme from cowpea. Arch. Biochem. Biophys. 226: 190–197.
- Kim, Y.R., Yu, S.W., Lee, S.R., Hwang Y.Y., Kang, S.O. (1996). A heme-containing ascorbate oxidase from Pleurotus ostreatus. J. Biol. Chem. 271: 3105–11.
- Ortega, N., Perez-Mateos, M., Pilar M.C., Busto, M.D (2009). Neutrase immobilization on alginate-glutaraldehyde beads by covalent attachment. J. Agric. Food Chem. 57: 109–115.
- Garg N., Kumar, A. (2008). Immobilization of starch phosphorylase from cabbage leaves: production of glucose-1–phosphate. Braz. J. Chem. Eng. 25: 229–235.
- Running J.A., Urdea, M.S. (1990). A procedure for productive coupling of synthetic oligonucleotides to polystyrene microtiter wells for hybridization capture. Biotechniques, 8: 276–277.
- Marques E.T., Lima-Filho J.L. (1992). Ascorbic Acid Biosensor Using Ascorbate Oxidase Immobilized on Alkylamine Glass Beads. Appl. Biochem. Biotechnol. 32: 73–78.
- Fatibello-Filho O., Vieira Iolanda da. C. (2000). L-ascorbic acid determination in pharmaceutical formulations using a biosensor based on carbon paste modified with crude extract of zucchini (Cucurbita pepo). J. Braz. Chem. Soc. 11: 412–418.
- Iqbal, K., Khan A., Khattak, M.A.K. (2004). Biological Significance of Ascorbic Acid (Vitamin C) in Human Health - A Review. Pakistan J. Nutr. 3: 5–13.