Abstract
Abstract: The effects of the distal nerve degeneration on the regeneration of the collateral sprouts from the proximal nerve stump have been examined. The delayed cross-suture anastomosis technique was used in which the tibial nerve was denervated for 0-8 weeks before cross-suture of the freshly axotomized common peroneal and chronically denervated TIB nerve stumps. There was a remarkable decreasing of the regenerated myelinated axons number after the distal nerve suffered 8 weeks deterioration, suggesting that short-term denervation did not affect the collateral sprouts regeneration but more prolonged denervation profoundly reduced collateral sprouts regenerated in the distal nerve stump.
INTRODUCTION
Peripheral nerve injuries induce severe disability and suffering for patients. Failure to restore the injured nerves can lead to loss of muscle function, impaired sensation, and/or painful neuropathies. Over the past century, there have been significant advances in human peripheral nerve repair. However, for severe peripheral nerve injuries, such as brachial plexus nerve injury and long nerve defect, they have been remedied by many traditional methods, like nerve transfer, nerve grafts, artificial nerve conduit bridging, and end-to-side neurorrhaphy, which are still suboptimal. So how to improve the treatment of severe peripheral nerve injury has became a concern. The outgrowth of collateral sprouts from injured nerve both on sensory and motor axons is a natural process that arises during regeneration in the peripheral nervous system. When a nerve is damaged, the proximal stump of damaged axons sends out numerous sprouts to reach endoneurial tubes in the distal nerve stump. Based on this behavior, once a small donor nerve is applied to repair a large receptor nerve, the donor nerve may send out more regenerative nerve fibers to grow into the receptor nerve, which obviously helps in the function recovery of the receptor nerve. In this case, there are many influencing factors in this nerve amplification, such as the ratio between donor nerves and receptor nerves, the regenerative ability of proximal donor nerves, and the reception ability of distal receptor nerves, etc. In this study, the effects of the distal nerve degeneration on the regeneration of the collateral sprouts from the proximal nerve stump were examined.
MATERIALS AND METHODS
Animals and Grouping
Male Sprague-Dawley rats (Vital River Laboratories, Beijing, China) that weighed 220-250 g were maintained under specific, pathogen-free laboratory conditions on a 12 h light/dark cycle with free access to pellet food and water. They were separated into 5 groups at random (6 animals in each group), which were named fresh-repaired group, 1-week group, 2-week group, 4-week group, and 8-week group. In the present work, every effort was made to minimize animal suffering and reduce the number of animals used, and the protocols were approved by the Committee of Animals Used for Research of People's Hospital.
Surgical Procedures
The delayed cross-suture anastomosis technique [Citation1] was performed on a rat whosetibial nerve was denervated for 0–8 weeks before cross-suture of the freshly axotomized common peroneal (CP) nerve and chronically denervated tibial (TIB) nerve stumps. Surgical procedures were carried out under a binocular surgical microscope, using a microsurgical technique. Rats were anesthetized with 2% sodium pentobarbital (30 mg/kg i.p.). After the anesthesia, right rat limbs were treated in a sterile manner. The sciatic nerve and its two main branches (the CP nerve and the TIB nerve) were exposed. The TIB nerve was transected at a level 5 mm distal to the bifurcation of the sciatic nerve, and the two nerve residuals were ligated with 10-0 nylon sutures, reversed and sutured together with nearby muscle. Then the wound was closed using 4-0 nylon sutures and the rats were sent back to their cages. After the surgical operation, according to various groups, rats were anesthetized and the right sciatic nerves were exposed again. The CP nerve was transected and half proximal residual was ligated. Subsequently, the proximal CP nerve residual, which served as the donor nerve, was fixed together with the distal degenerative TIB nerve stump with 1 mm gap. For this, biodegradable chitin conduits were used (patented by our laboratory and authorized by the State Intellectual Property Office of the People's Republic of China No. ZL01 136314.2; this was an artificial nerve graft consisting of shell polysaccharide that showed satisfactory biocompatibility and degradation characteristics; this conduit is now in a preclinical study) [Citation9–10]. The biodegradable chitin conduits were 4 mm long, 0.5 mm thin, with an inner diameter of 1.5 mm. Finally, the wound was closed and the rats were sent back to their cages.
Detection Project
Electrophysiology. Electrophysiological assessment was performed 3 months postsurgically, prior to sacrifice of the animals. Rats were first anesthetized with 2% sodium pentobarbital (30 mg/kg i.p.). The right repaired TIB nerves were exposed and the recording electrode was placed in the gastrocnemius muscle, while the ground electrode was placed subcutaneously. The stimulating bipolar electrodes were placed on the proximal and distal repair site in each group. Rectangular pulses (duration 0.1 ms, 0.9 mA) were used to stimulate the repaired nerves. Compound muscle action potential (CMAP) was recorded and motor nerve conduction velocity (MNCV) was obtained simultaneously by dividing the distance between the two stimulating sites by the difference in the onset latency.
Muscle histology study. To study the muscle general morphology, rats were sacrificed by an intra-arterial overdose (2 ml) of sodium pentobarbital solution (30 mg/kg), and then both sides of tibialis anterior muscle were harvested and rinsed with PBS and fixed in 4% paraformaldahyde solution for histological assessment. Fixed specimens were paraffin embedded; 5 mm sections were cut and stained with hematoxylin and eosin (H&E) according to Harris. Slides were examined under light microscope.
Nerve histological study and myelinated nerve fibers counting. For histological study, the entire TIB nerve, including the repaired segment, was removed en bloc from each rat. Tissues were harvested and fixed in 4% paraformaldehyde in 0.1 M phosphate buffer for 48 h at 4°C. After this, the nerves were rinsed twice in phosphate buffer, and 3 tissue blocks (approx 5mm long) were cut, one proximal to the residual segment, one distal to the residual segment, and one from the residual segment. After this step, each sample was fixed in 1% osmium tetroxide for 12 h, dehydrated through a graded series of ethanol, and embedded in paraffin. Specimen sections were then taken for osmium acid staining of the entire nerve perpendicular to the long axis of the nerve fibers. Myelinated axons were quantified according to the unbiased counting rule. Finally, the total number of myelinated axons was counted using Imagetool image analytical software. The amplification ratio was obtained from dividing the regenerative myelinated nerve fibers by myelinated nerve fibers of donor.
Statistical Analysis
The results were expressed as mean ± standard deviation. The differences among several groups were evaluated with one way ANOVA. Analysis was performed by using the SPSS version 13.0 software (SPSS Inc., Chicago, IL, USA). P value less than 0.05 was considered statistically significant.
RESULTS
In all animals, the surgical procedures were well-tolerated and wounds healed without infection. Ulcers appeared at paws of experimental TIB nerve on 3 rats in degenerative groups, the 18th (4-weeks group), 18th (8-weeks group), and 23rd (8-weeks group) day after surgery, respectively.
Electrophysiology
The MNCV of regenerated TIB nerve were 23.09 ± 2.79 m/s (fresh-repaired group), 24.03 ± 0.95 m/s (1-week group), 23.44 ± 3.01 m/s (2-week group), 21.23 ± 2.24 m/s (4-week group), 16.99 ± 3.74 m/s (8-week group), respectively (). There were no statistical differences among the fresh-repaired group, 1-week group, 2-week group, and 4-week group. However, the MNCV of the 8-week group was obviously lower than the other groups.
Table 1. Electrophysiology and myelinated nerve fibers counting
Muscle Histology Study
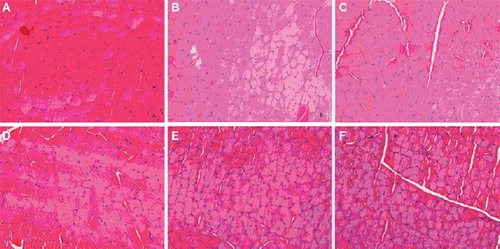
Cross-sections demonstrated muscles of the experimental side were various degrees atrophy with smaller diameter muscle fiber compared with the one of the control side (). The average diameters of the muscle fiber were progressively reduced when the distal nerve stump suffered 0-8 weeks degeneration before repair.
Figure 1. Images of the transverse section of the Tibialis anterior muscle (Hematoxylin-Eosin stain, X400), compared to the normal muscle of the contralateral control side (A); muscles of the experimental side revealed atrophy with the average diameter of the muscle fiber progressively reduced when the distal nerve stump suffered 0–8 weeks degeneration before repair: fresh-repaired (B); 1-week degeneration (C); 2-weeks degeneration (D); 4-weeks degeneration and 8-weeks degeneration (E).
Nerve Histology Study and Myelinated Nerve Fibers Counting
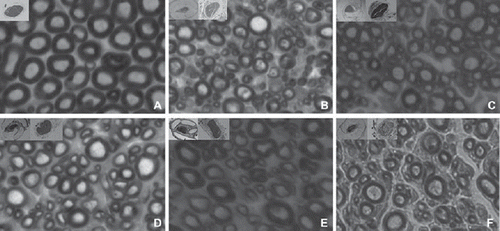
The transverse section of both the regenerated distal TIB nerve in every group and the normal TIB nerve in the contrary side were examined at light microscopic level (). Compared to the myelinated axons in the normal TIB nerve, which were approximately round and equal in diameter, myelinated axons in the regenerated TIB nerve had varied diameters from 2μm to 10μm with a thinner myelin sheath. The regenerated myelinated axons of each group were similar in morphology. However, the density of the regenerated myelinated axons in the 8-week group was lowest. The average of total myelinated fiber count from donor nerve in the fresh-repaired group, 1-week group, 2-week group, 4-week group, and 8-week group were 1285.84 ± 302.11, 1278.51 ± 284.55, 1304.00 ± 415.11, 1227.84 ± 372.24, 1253.34 ± 284.21, and the regenerated nerve were 3031.17 ± 421.34, 2979.34 ± 349.53, 3025.34 ± 407.52, 2761.00 ± 396.58, 1816.34 ± 276.35, respectively. Thus, the average amplification ratios were 2.41, 2.33, 2.32, 2.25, 1.45, respectively (). There were no statistical differences among these groups except the average amplification ratio of the 8-week group was obviously lower than the others.
Figure 2. Images of the transverse section of the TIB nerve (stained by osmium tetroxide, X1000): the contralateral control side (A); the experimental side of the fresh-repaired group (B); the experimental side of the 1-week group (C); the experimental side of the 2-week group (D); the experimental side of the 4-week group and the experimental side of the 8-week group (E).
DISCUSSION
During the regeneration of peripheral nerve, there is a behavior of amplification. It has been shown that the axons send out many collaterals that grow into the target tissue. Sprouts from the node of Ranvier extend through their own basal lamina tubes in the proximal segment, and then enter the distal nerve segment. The evidence shows that the maximum amplifying ratio for immediately repaired sciatic nerve of rats is 3.3 [Citation2]. Namely, once the distal nerve stump is big enough, one proximal nerve fiber can send and support maximally three to four collaterals into the distal nerve stump. Based on this theory, in the case of repairing large and important injured peripheral nerves with absent proximal nerve stump, such as the patient with brachial plexus nerve injury and long nerve defect, a small or thin unnecessary donor nerve could play a role [Citation3–4].
Apart from the ratio of donor nerve fiber and receptor nerve fiber, and regenerative ability of proximal donor nerve, the reception ability of the distal nerve is one important impact factor for the amplification of the nerve fiber. After injury, there will be a series of special changes with the distal nerve, namely Wallerian degeneration, including the collapse of original distal nerve fiber, the proliferation of Schwann cells, and the formation of Bung band, and finally leads the regenerative nerve fibers to grow into. The reception ability of degenerated nerves for regenerative nerve fibers varies from the periods of degeneration. Olawale et al. showed that the reception ability of distal nerves reduced significantly when they had been degenerating for 8 weeks [Citation1] mean period, and no significant change with the myelinization ability of regenerative nerve fiber. According to this study, the amplification ratio reduced markedly when repairing the nerves with degeneration of 8 weeks. To explain this, the tissue structure in the degenerated distal nerve was destroyed gradually within the degeneration, and the Schwann cells atrophied to apoptosis [Citation5–6]. Also, there were fewer nerve growth factors excreted by cells in the long-term degenerated distal nerve [Citation7]. Finally, due to limitations of cell metabolism, there was no adequate supply of necessary constituents for such many sprouts to grow into the degenerated nerve [Citation8].
The rat sciatic nerve model is a widely used model for the simultaneous evaluation of nerve function. In this study, the CP nerve as donor nerve was used to form a 3 to 1 ratio between receptor nerve and donor nerve. In this case, there were enough spaces in the distal nerve for the regenerative proximal nerve to grow into, and the amplification ratio was approximately the maximum, which would impersonally reflect the influence of various degeneration periods to amplification of donor nerves. On the other hand, because of the obvious difference between the donor nerve and receptor nerve, the biodegradable chitin conduits was adopted to suture, which made suture easier and, importantly, reduced the escape of regenerative nerve fibers to obtain a better amplification effect [Citation9–12]. In addition, traditional methods of assessing nerve recovery following peripheral nerve injury and repair, such as electrophysiology and histomorphometry, were included in the present study. It demonstrated that within 4 weeks degeneration of the distal receptor nerve, there was no significant change for the amplifying ratio of the proximal donor nerve. Nevertheless, the amplifying ratio decreased markedly with the degeneration of 8 weeks, which offered an experimental basis for clinically choosing the nerve repair period. On the other hand, long-term degeneration of the distal receptor nerve went against the growth of the regenerative donor nerve, thus extending the degeneration and keeping the reception ability of the distal receptor nerve would help the nerve repair based on the amplification theory.
Declaration of interest: This research project was funded by the Chinese National Natural Science Fund for Outstanding Youth (30625036), Chinese 973 Project Planning (2005CB522604), Chinese National Natural Science Youth Fund (30801169). Chinese National Natural Science Fund (30971526), Beijing City Science and Technology New Star Classification (A 2008 10), Chinese Ministry of Education for Doctor Position New Teacher (20070001780), and Project (RDB2009 01), supported by Peking University People's Hospital Research and Development Funds. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Olawale, A.R., Sulaiman Gordon, T. (2000). Effects of short- and long-term Schwann cell denervation on peripheral nerve regeneration, myelination, and size. Glia. 32: 234–246.
- Jiang, B., Yin, X., Zhang, D., Fu, Z., Zhang, H. (2007). Maximum number of collaterals developed by one axon during peripheral nerve regeneration reinnervation effects. European Neurology. 58: 12–20.
- Schessler, M.J., McClellan, W.T. (2010). The role of nerve transfers for C5-C6 brachial plexus injury in adults. West Virginia Medical Journal. 106(1): 12–7.
- Dahlin, L., Johansson, F., Lindwall, C., Kanje, M. (2009). Future perspective in peripheral nerve reconstruction. Int Rev Neurobiol. 87: 507–30.
- Giannini, C., Dyck, P.J. (1990). The fate of Schwann cell basement membranes in permanently transected nerves. J Neuropathol Exp Neurol. 49: 550–563.
- Wood, P.M., Cuervo, E.F., Bunge, R.P., Gordon, T. (1998). Functional capacities of long-term denervated Schwann cells. Soc Neurosci Abs. 24: 690–698.
- Shamash, S., Reichert, F., Rotshenker, S. (2002). The cytokine network of wallerian degeneration: tumor necrosis factor-a, interleukin-1a and interleukin-1h. J. Neurosci. 22: 3052–3060.
- Gordon, T., Fu, S. (1997). Long-term response to nerve injury. Adv Neurol. 72: 185–199.
- Sun, Y., Jiang, B., Zhu, Q. (2002). The experimental functional medical material in animal body. Spinning and Weaving Science Research. 13: 7–22.
- Jiang, B., Zhang, P., Zhang, D., Fu, Z., Yin, X., Zhang, H. (2006). Study on small gap sleeve bridging peripheral nerve injury. Artif Cells Blood Substit Immobil Biotechnol. 34(1): 55–57.
- Jiang, B., Zhang, P., Jiang, B. (2010). Advances in small gap sleeve bridging peripheral nerve injury. Artif Cells Blood Substit Immobil Biotechnol. 38(1): 1–4.
- Zhang, P., Kou, Y., Yin, X., Wang, Y., Zhang, H., Jiang, B. (2010). The experimental research of nerve fibers compensation amplification innervation of ular nerve and musculocutaneous nerve in rhesus monkeys. Artif Cells Blood Substit Immobil Biotechnol.