Abstract
Abstract: Encapsulation of cells in polymeric shells allows for separation of biological material from produced factors, which may find biotechnological and biomedical applications. Human T-lymphocyte cell line Jurkat as well as rat pancreatic islets were encapsulated using LbL technique within shells of polyelectrolyte modified by incorporation of biotin complexed with avidin to improve cell coating and to create the potential ability to elicit specific biochemical responses. The coating with nano-thin modified shells allowed for maintenance of the evaluated cells’ integrity and viability during the 8-day culture. The different PE impact may be observed on different biological materials. The islets exhibited lower mitochondrial activity than the Jurkat cells. Nevertheless, coating of cells with polyelectrolyte modified membrane allowed for functioning of both model cell types: 10 μm leukemia cells or 150 μm islets during the culture. Applied membranes maintained the molecular structure during the culture period. The conclusion is that applied modified membrane conformation may be recommended for coating shells for biomedical purposes.
INTRODUCTION
Encapsulation of cells in membranes allows for separation of biological material from produced factors, which may find straightforward biotechnological application. Such a system may serve for immunoisolation, allowing for interspacing encapsulated cells from the host immunological system in biological processes’ regulation in biomedical applications.
Cells may be encapsulated using different techniques and devices like diffusive chambers, macrocapsules, and microcapsules. A special case of microencapsulation, conformal coating, is a method of encapsulation allowing the minimization of capsule void volume by deposition coatings that conform to the surface of cells, generating layers on geometrically diverse substrates [Citation1–2]. This form of encapsulation finds several applications in systems for regulation of biological processes using, cells as the encapsulated material, e.g. mouse mesenchymal stem cells [Citation4], erythrocytes [Citation5], yeast [Citation6, Citation7], E. coli cells [Citation8], DNA [Citation9], drugs [Citation10], and proteins [Citation11, Citation12]. Conformal coating may be performed by layer-by-layer technique (LbL) using self assembly processes of polymers involving electrostatic interactions. The alternate deposition of oppositely charged polyions onto charged substrates allows the build-up of polyelectrolyte multilayers. Different membrane materials have been applied to form the layers, e.g. poly(styrene sulfonate) and poly(allylamine), hyaluronic acid and poly(L-lysine), poly(ethylene glykol) and alginate and poly(L-lysine) [Citation13], poly(ethylene glycol) and poly(vinyl alcohol) [Citation14]. Coating properties may be tailored [Citation15, Citation16] for different purposes, e.g. inhibition of molecular recognition between complementary molecules or to enhance adsorption to target cells.
The suitability of modified polyelectrolyte (PE) coatings for biological material encapsulation was assessed. An additional interaction between PE layers has been introduced by using ligand derivatized polymers creating an interface capable of binding receptors. We demonstrated the ability to individually encapsulate the human T-lymphocyte cell line Jurkat or rat pancreatic islets within ligand derivatized polymeric shells to increase the layers’ stability and to create the potential ability to elicit specific biochemical responses, using the LbL technique as a model system of encapsulated in membrane biological material for applications in biological processes’ regulation.
MATERIALS AND METHODS
Materials
Reagents: poly-L-lysine hydrobromide (MW 15-30kD) (Sigma, USA), poly(ethylenimine) (MW 60 kD) (Aldrich, USA), D-biotin (Sigma, USA), avidin FITC conjugate (Sigma, USA); (3-4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) (Sigma, USA); Live/ Dead® Viability/Cytotoxity Kit (Molecular Probes, USA).
Media: RPMI-1640 (Biomed, Poland); newborn calf serum NCS (Biochorom, UE); Hanks (Wytwórnia Surowic i Szczepionek, Poland); Histopaque ρ = 1,083 (Sigma, USA).
Culture media: RPMI-1640 supplemented 10% NCS (RPMI-1640/10%NCS).
Cell line: Jurkat – human T-lymphocyte cell line.
Islets of Langerhans – isolated from pancreas of rats WAG.
Procedures
Biotin-labeled Polymers Preparation
Biotin-labeled polymers were prepared by the reaction of the poly(amine)s with a calculated amount of biotin. To obtain primary and secondary amino groups in the polymer chain, random substitution through amido linkage with biotin residues was applied, and the mixture of 10 mg of polyethyleneimine (PEI) or polylysine (PLL) and 4 mg of biotin in 10 ml of 0.1 M NaCl (pH 7.2) was stirred and incubated for 24 hours at room temperature. Then the modified PEI or PLL was purified by centrifugal filtration and polyelectrolytes with incorporated biotin (PEbiot). PEIbiot or PLLbiot were obtained.
Preparation of Complexes with Polyelectrolytes
To obtain the biotin-labeled PLL complex with avidin (PLLcx), the biotin-labeled PLL (PLLbiot) was stirred with 0,5% avidin solution in 0.1 M NaCl (pH 7.2) in proportion 1:10 and then incubated in 4°C during the night.
Coating of Jurkat Cells with Polyelectrolytes
Cationic layer deposition. 5 × 106 Jurkat cells were incubated with 0,1% PLL or PLLcx solution in 0,1 M NaCl. After 5 min deposition time cells were washed two times with RPMI-1640 at 1000 rpm for 3 minutes to remove unadsorbed polyelectrolyte.
Anionic layer deposition. Encapsulated in polycation Jurkat cells were incubated with 0,1% PEI or PEIbiot solution in 0,1 M NaCl. After 4 min deposition time they were washed two times with RPMI-1640 at 1000 rpm for 3 minutes to remove uncoupled polyelectrolyte.
The process was repeated to obtain more than one bilayer consisting of a cationic and anionic layer. The double bilayers were applied: polylysine and polyethyleneimine (PLL/PEI) or PLLbiot complexed with avidin and PEbiot (PLLcxPEI).
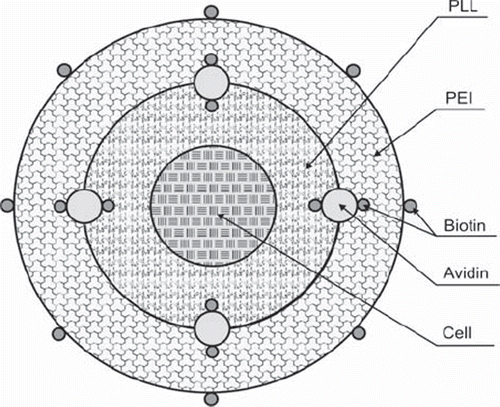
The avidin binding by derivatized polyelectrolytes (PE) was applied to involve bioconjugation simultaneously with electrostatic interactions between the PE layers to improve the biological material covering by consecutive layers, increasing the layers’ stability as well as enabling the surface selectivity of reactions with potential target cells. The scheme of the shells covering biological material is presented in . There is avidin bound by incorporated in the layers biotin involved in the shells.
Rat Pancreatic Islets Isolation
Rat pancreatic islets were isolated using a modified Lacy and Kostianovsky method [Citation17] by intraductal injection of 10 ml of 1000U/ml collagenase solution in Hanks/ 1%FCS to a common bile duct before pancreas resection. Pancreas digestion was performed in a water bath at 37°C by putting it onto plastic Petrischale. Then the tissue was transferred to a 50 ml centrifugation tube containing 20 ml of cold Hanks/1%FCS. The enzyme was washed out twice by Hanks/1%FCS (4°C, 600 × g, 5 minutes). The digested material was filtered through a sterile mesh (500-nm pores), transferred to a 10 ml centrifugation tube filled with Hanks 1%FCS, and centrifugated (600 × g, 5 minutes). Islet purification was performed by discontinuous density gradient centrifugation with 1,083 g/ml Histopaque. Then the islets were washed by Hanks/1%FCS (800 × g, 16 min.) and suspended in culture medium RPMI-1640/10%FCS at pH = 7.4.
Coating of Rat Pancreatic Islets with Polyelectrolytes
250 rat pancreatic islets of Langerhans were incubated in polyelectrolyte suspension as described for Jurkat cells to deposit polyelectrolyte layers.
Encapsulated cells (Jurkat or islets) were cultured (37°C, 5%CO2) in culture medium RPMI-1640/10%NCS for 8 days. As a negative control, non-encapsulated cells were cultured (37°C, 5%CO2) in culture medium RPMI-1640/10% NCS for 8 days. The mitochondrial activity of cells was measured after 1, 5, or 8 days of culture in MTT test. The yield of nanocapsules was assessed as well. The viability of cells was examined using a LIVE/DEAD® Viability/Cytotoxity Kit containing calcein-AM and ethidium homodimer-1.
The PE layers’ presence was evaluated using FTIR techniques. The avidin presence in the complex with PEbiot layer was assessed using confocal microscopy.
MTT Test
The (3-4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed. Encapsulated cells were seeded at a density of 10000 Jurkat cells or 10 islets per well in a 96-well plate and incubated in a culture medium RPMI-1640/20%FCS or RPMI-1640/10%NCS, respectively. The MTT solution in concentration 5mg/ml was added to the culture in a 1:10 dilution of the medium and 2-hour incubation was performed. Then the suspension was centrifuged (1000 rpm, 3min). The formazan crystals were dissolved with 100 μl DMSO added to the pellet. After 10 minutes of shaking an absorbance was measured at 540 nm in spectrophotometer.
Confocal Laser Scanning Microscopy
Image acquisition was performed using a confocal spectral microscope. Images were collected with a 100 × oil NA = 1.4 objective. Green fluorescence of FITC was excited with an argon ion (488 nm) laser beam. Emission filter 500 LP (long pass) was applied. All resulting images were acquired, stored, and visualized with the Leica confocal software (LCS).
Spectroscopic Evaluation
The PE layers’ presence was assessed by evaluation of spectrum of absorption for red irradiation (FTIR) before and after an 8-day culture. The FTS3000MX (BioRadExcalibur, USA) device was applied. The 16 scans were collected with resolution 4 cm−1.
AFM Measurements
A Nanoscope V AFM microscope (Veeco Instruments, Inc.) was used in imaging the surface of the samples. The silicon nitride cantilevers with a spring constant ca. 0.06 Nm−1 (manufacturer data) were applied for imaging in Contact Mode AFM (CM-AFM). Presented images were visualized in dedicated Nanoscope software.
Encapsulated cells were visualized in 3D form in dedicated Nanoscope software. Cells were placed on the Nucleopore polycarbonate membrane (Whatman Ltd.) of 800 nm pore size by the gravity drying method. Contact mode AFM images were obtained at room temperature.
The samples for AFM evaluation were prepared by preservation in ethanol. The procedure was as follows.
Preservation in Ethanol
To 1ml Falcon test tube with 1ml physiological saline supplemented with 1% formalin 50 μl of evaluated cells suspension was added and incubated for 10 minutes. Then the mixture was centrifuged (1200 rpm, 3 min), and the supernatant was removed. The pellet was added to 2 ml of cooled 70% ethanol and incubated for 10 minutes in ice.
Fluorescent Staining of Cells
A LIVE/DEAD® Viability/Cytotoxity Kit containing calcein-AM and ethidium homodimer-1 (Molecular Probes, USA) was used to examine the viability of cells. The kit was used according to the producer's recommendations. The preparations were covered with a cover glass and observed with a fluorescence microscope IX70 (Olympus).
Visualization and Analysis of Results
For fluorescence observation, the light passing through the double filter FITC-TRITC, with a wavelength of 475-490 nm and 540-565 nm, was used (Olympus IX70). Photographs were taken using a digital camera, Camedia C 5050 Olympus. Microphotographs were projected, visualized, and analyzed with DP-Soft 3.2 (Build 779) (analySIS®) software produced by Soft Imaging Systems GmbH for Olympus or ImageJ (Java-based image processing program developed at the National Institutes of Health), and the final montage was created with Adobe Photoshop, version 7.0.
RESULTS
The suitability of modified PE coatings for biological material encapsulation was assessed. As model cells, leukemia cells of about 10 μm diameter and rat islets about 150 μm were used. The modified layers were applied to improve layer stability by reinforcing the binding between the layers as well as for further development for adsorption with potential target cells. For that purpose, binding of avidin with biotin incorporated in a nanocoating layer was used.
Assessing the mean yield of capsules performed with PLL/PEI or PLLcxPEI, it was observed that the mean yield of nanocapsules obtained for PLLcxPEI deposition on Jurkat cells or islets was about 20% lower as compared to PLL/PEI deposition.
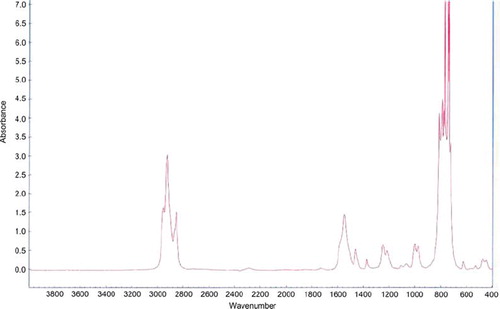
FTIR spectrum evaluation allows us to assess the PE layer presence and its chemical stability during the culture. The FTIR spectrum for layers PLL/PEI as well as for PLLcxPEI was evaluated before and post 8-day culture. The extract of evaluated membrane was analyzed. FTIR evaluation in spectrum 4000-666 [cm−1] did not exhibit chemical changes in evaluated membranes structure after a short 8-day culture. The layers’ presence was confirmed by characteristic picks existence at frequency [cm−1]: 2917 (exhibiting CH2 presence); 1597, 1249 (exhibiting NH2 group presence), 1052 (exhibiting N-C-N binding presence) ().
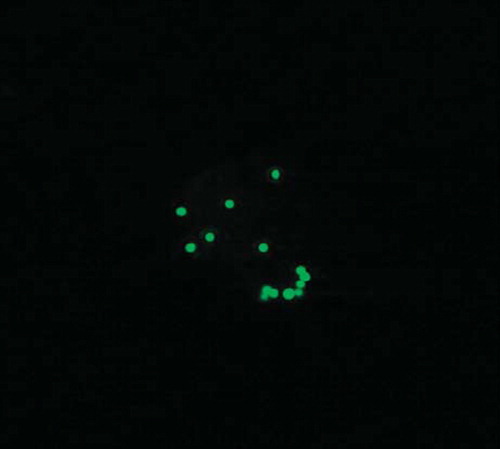
The avidin presence in complex with a PEbiot layer on the assessed cell's surface was confirmed using visualization in a confocal microscope. In is presented about 10 μm of diameter Jurkat cell coated with a layer of PLLbiot complexed with avidin. The avidin FITC conjugate is visible as the single dots on the spherical surface of the Jurkat cell.
Figure 3. Confocal visualization of Jurkat cells coated with one layer PLLbiot complexed with avidin FITC (×3000).
The AFM visualization of a Jurkat cell coated with bilayer PLLcxPEI is presented in . Single PE layer thickness is about 17 nm according to the profiles of images analysis.
Figure 4. AFM image of Jurkat cell coated with PLLcxPEI shell, deposited on filtration membrane (Nanoscope V, Veeco Instruments, Inc., USA).

Cell viability assessment was made on the basis of a set of photos of preparations stained using the LIVE/DEAD® Viability/Cytotoxity Kit. Live cells were distinguished by the presence of ubiquitous intracellular esterase activity, determined by the enzymatic conversion of the virtually nonfluorescent cell-permeant, a lipid-soluble fluorogenic diester calcein AM, to the intensely fluorescent calcein. Calcein AM, crossing the cell membrane in electrically neutral form, stands out as the premier indicator of cell viability due to its superior cell retention and the relative insensitivity of its fluorescence to pH in the physiological range [Citation18, Citation19, Citation20].
With its high affinity for DNA and low membrane permeability, EthD-1 enters cells with damaged membranes and undergoes a 40-fold enhancement of fluorescence upon binding to nucleic acids, thereby producing a bright red fluorescence in dead cells (495nm/635nm) [Citation21, Citation22, Citation23]. EthD-1 is excluded by the intact plasma membrane of live cells. Photographs were taken after 8-day culture.
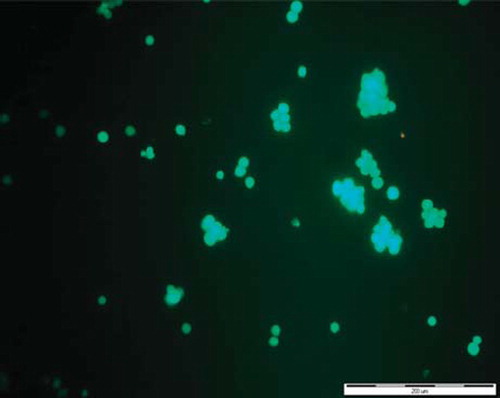
Both types of cells encapsulated using layers PLL/PEI or PLLcxPEI exhibited retained dye calcein. An intensive, uniform green fluorescence in live cells was observed after 8-day culture. Absence of red fluorescence suggested that the cell plasma membrane is intact. In , visualization of the Jurkat cells coated with PLLcxPEI double bilayer after 8-day culture in a fluorescence microscope is presented.
Figure 5. Fluorescence microscopy visualization of Jurkat cells coated with two bilayers, PLLcxPEI, after 8-day culture. Encapsulated cells exhibited retained dye calcein.
The mitochondrial activity of leukemia Jurkat cells, encapsulated in double bilayers PLL and PEI with biotin-avidin complex involvement (PLLcxPEI) or double bilayers PLL/PEI, was assessed in MTT test. The values obtained for these two types of polyelectrolyte layers were comparable with the control on 5 and 8 days of culture. It was observed that there were no differences in mitochondrial activity of Jurkat cells coated with PLL/PEI or PLLcxPEI shells after 8-days culture. The Jurkat cells coated with one layer PLL with incorporated biotin and complexed with avidin (PLLcx) exhibited lower mitochondrial activity then those encapsulated in bilayers cells during the whole period of culture ().
Figure 6. Evaluation of the mitochondrial activity in MTT test of encapsulated Jurkat cells during 8-day culture: The ratio of absorbance for cells coated with polyelectrolyte layers to the negative control absorbance. PLL/PEI – absorbance of cells encapsulated with double bilayer of PLL and PEI double bilayer; PLLcxPEI – absorbance of cells encapsulated with PLL with incorporated biotin complexed with avidin and PE with incorporated biotin; CONTROL- non-encapsulated cells’ absorbance.
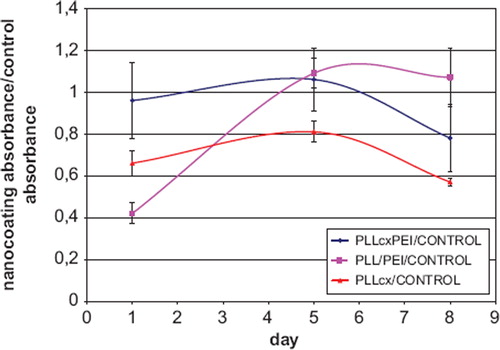
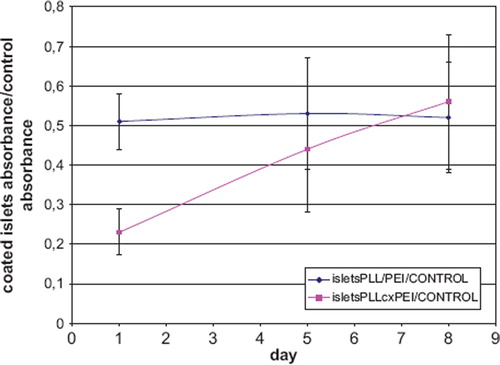
The islets encapsulated within the PLL/PEI coating exhibited mitochondrial activity two times lower as compared to a negative control. In the case of the islets coated with PLLcxPEI shells, mitochondrial activity increase was observed during the culture up to 60% activity of control cells on day 8 ().
Figure 7. Evaluation of the mitochondrial activity of encapsulated islets during 8-day culture in MTT test: The ratio of absorbance for cells coated with PE to the negative control absorbance. isletsPLL/PEI – absorbance of islets encapsulated within double bilayer of PLL and PEI; isletsPLLcxPEI – absorbance of islets encapsulated with double bilayer of PLL with incorporated biotin complexed with avidin and PEI with incorporated biotin; CONTROL – non-encapsulated islets’ absorbance.
DISCUSSION
In our approach, biotin molecules were conjugated to amino groups of PE, which were complexed by avidin for construction of a number of consecutive layers.
The coating with nano-thin modified shells produced without covalent interaction using electrostatic interactions and bioconjugation forces allowed for maintenance of the evaluated cells’ integrity and viability during 8-day culture. The different PE impact may be observed on different biological material. Nevertheless, coating of cells with polyelectrolyte modified bilayers PLLcxPEI allowed for functioning of both model cell types: 10μm leukemia cells or 150 μm islets during the culture. Applied membranes maintained molecular structure during the culture period. In addition, long-term stability of the PLLcxPEI membrane and its immunoisolation efficacy should be examined and reported in near future.
Application of the polymers able to create interfaces capable of binding biologically active solutes may be a way to obtain coatings elicting specific biochemical response, e.g. membranes carrying urokinaze in purpose to decrease fibrotic reaction, which has been reported to retain activity during 3-day culture in vitro [Citation24] also membranes modified for enhanced ligand absorbance to target cells [Citation25] in anti-cancer therapy.
This paper presents a method of shell modification allowing for construction of a surface capable of creating the potential ligand affecting the target cell without reducing cell viability or the capsule void volume increase, which seems to be a promising solution for biomedical applications.
CONCLUSIONS
We demonstrated the ability to individually encapsulate the human T-lymphocyte cell line Jurkat as well as rat pancreatic islets within modified polymeric shells using the LbL technique as a model system of biological material encapsulated in membrane shells for applications in biological processes regulation. Applied membranes maintained molecular structure during the culture period. The PLLcxPEI conformation, allowing for survival and functioning of encapsulated model cells, may be recommended for coating shells for biomedical purposes.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Decher, G. (1997). Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science. 277: 1232–1237.
- Quinn, J.F., Johnston, A.P.R, Such, G.K., Zelikin, A.N., Caruso, F. (2007). Next generation, sequentially assembled ultrathin films: beyond electrostatics. Chem. Soc. Rev. 36:707–718.
- Hammond, P.T. (2004). Adv. Mater. 16: 1271–1293.
- Veerabadran, N.G., Goli, P.L., Stewart-Clark, S.S., Lvov, Y.M., Mills, D.K. (2007). Nanoencapsulation of stem cells within polyelectrolyte multilayer shells. Macromol. Biosci. 7: 877–882.
- Donath, E., Moya, S., Neu, B., Sukhorukov, G.B., Georgieva, R., Voigt, A., Baumler, H., Kiesewetter, H., Mohwald, H. (2002). Hollow polymer shells from biological templates: Fabrication and potential applications. Chem. Eur. J. 8: 5481–5485.
- Diaspro, A., Silvano, D., Cavalleri, O., Gliozzi, A. (2002). Living cell encapsulation in nano-organized polyelectrolyte shells. Langmuir 18: 5047–5050.
- Svaldo-Lanero, T., Krol, T., Magrassi, R., Diaspro, A., Rolandi, R., Gliozzi, A., Cavalleri, O. (2007). Morphology, mechanical properties and viability of encapsulated cells. Ultramicroscopy 107: 913–921.
- Hillberg, A., Tabrizian, M. (2006). Biorecognition through Layer-by-Layer Polyelectrolyte Assembly: In-Situ Hybridization on Living Cells. Biomacromol. 7: 2742–2750.
- Jewell, C.M., Zhang, J.T., Fredin, N.J., Lynn, D.M. (2005). Multilayered polyelectrolyte films promote the direct and localized delivery of DNA to cells. J. Contr. Rel. 106: 214–223.
- Benkirane-Jessel, N., Schwinte, P., Falvey, P., Darcy, R., Haikel, Y., Schaaf, P., Voegel, J.C., Ogier, J. (2004). Build-up of polypeptide multilayer coatings with anti-inflammatory properties based on the embedding of piroxicam-cyclodextrin complexes. Adv. Funct. Mater. 14: 174–182.
- Lvov, Y., Ariga, K., Ichinose, I., Kunitake, T. (1995). Assembly of Multi-component Protein Films by Means of Electrostatic Layer-by-Layer Adsorption. J. Am. Chem. Soc. 117: 6117–6123.
- Jessel, N., Atalar, F., Lavalle, P., Mutterer, J., Decher, G., Schaaf, P., Voegel, J.C., Ogier, J. (2003). Bioactive coatings based on a polyelectrolyte multilayer architecture functionalized by embedded proteins. Adv. Mater. 15: 692–695.
- Miura, S., Teramura, Y., Iwata, H. (2006). Encapsulation of islets with ultra-thin polyion complex membrane through poly(ethylene glycol)-phospholipids anchored to cell membrane. Biomater. 27: 5828–5835.
- Teramura, Y., Kaneda, Y., Iwata, H. (2007). Islet-encapsulation in ultra-thin layer-by-layer membranes of poly(vinyl alcohol) anchored to poly(ethylene glycol)-lipids in the cell membrane. Biomater. 28: 4818–4825.
- Picart, C., Elkaim, R., Richert, L., Audoin, T., Arntz, Y., Cardoso, M.D., Schaaf, P., Voegel, J.C, Frisch, B. (2005). Primary cell adhesion on RGD-functionalized and covalently crosslinked thin polyelectrolyte multilayer films. Adv. Funct. Mater. 15: 83–94.
- Chluba, J., Voegel, J.C., Decher, G., Erbacher, P., Schaaf, P., Ogier, J. (2001). Peptide hormone covalently bound to polyelectrolytes and embedded into multilayer architectures conserving full biological activity. Biomacromol. 2: 800–805.
- Lacy, P.E., Kostianovsky, M. (1967). Method for the isolation of intact islets of Langerhans from rat pancreas. Diabetes. 16: 35.
- Wang, X.M., Terasaki, P.I., Rankin, G.W., Chia, D., Zhong, H.P., Hardy, S. (1993). A new microcellular cytotoxicity test based on calcein AM release. Hum. Immunol. 37: 264–270.
- Neri, S., Mariani, E., Meneghetti, A., Cattini, L., Facchini, A. (2001). Calcein-acetoxymethyl cytotoxicity assay: Standardization of a method allowing additional analyses on recovered effector cells and supernatants. Clin. Diagn. Lab. Immunol. 8: 1131–5.
- Chiu, V.C., Haynes, D.H. (1977). High and low affinity Ca2+ binding to the sarcoplasmic reticulum: use of a high-affinity fluorescent calcium indicator. Biophys. J. 18: 3–22.
- Gaugain, B., Barbet, J., Oberlin, R., Roques, B.P., Le Pecq, J.B. (1978). DNA bifunctional intercalators. I. Synthesis and conformational properties of an ethidium homodimer and of an acridine ethidium heterodimer. Biochemistry 17:5071–5078.
- Gaugain, B., Barbet, J., Capelle, N., Roques, B.P., Le Pecq, J.B. (1978). DNA Bifunctional intercalators. 2. Fluorescence properties and DNA binding interaction of an ethidium homodimer and an acridine ethidium heterodimer. Biochemistry 17: 5078–5088.
- Lévesque, A., Paquet, A., Pagé, M. (1995). Measurement of tumor necrosis factor activity by flow cytometry. Cytometry 20: 181–184.
- Teramura, Y., Iwata, H. (2008). Islets surface modification prevents blood-mediated inflammatory responses. Bioconjugate Chem. 19: 1389–1395.
- Pulkkinen, M., Pikkarainen, J., Wirth, T., Tarvainen, T., Haapa-Acho, V., Korhonen, H., Seppala, J., Jarvinen, K. (2008). Three-step tumor targeting of paclitaxel using biotinylated PLA-PEG nanoparticles and avidin-biotin technology: Formulation development and in vitro anticancer activity. Eur. J Pharm Biopharm. 70: 66–74.