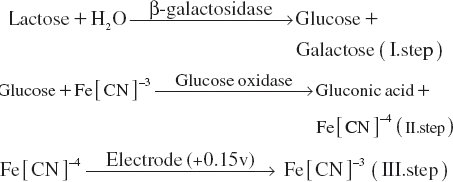Abstract
Abstract: β-galactosidase splits lactose into glucose and galactose. Because of its biotechnological interest, we presented a biosensor system in order to monitor β-galactosidase activity. Immobilization steps of the biosensor were identified by cyclic voltammograms and electrochemical impedance spectroscopy. β-galactosidase was voltammetrically detected at about +150 mV (vs. Ag/AgCl) in citrate buffer solution (0.05 M, pH 4.8). The linear response for β-galactosidase detection was in the range of 0.0118 U mL−1to 0.47 U mL−1and a shorter response time of ∼50 s. Our results demonstrated the biosensor's electrochemical properties and analytical characteristics were very useful and effective for monitoring of β-galactosidase activity.
INTRODUCTION
β-galactosidase (β-d-galactoside galactohydrolase, EC 3.2.1.23) from several sources, the enzyme that is responsible for the hydrolysis of lactose into glucose and galactose, has a very important biotechnological interest. Researchers, scientists, and industries pay attention to β-galactosidase studies because of their important applications in the fields of medicine (treatment of lactose intolerance), food technology (to prevent lactose crystallization and increase its sweetening power), and the environment (cheese whey utilization).
Electrochemical impedance spectroscopy (EIS) has been proven as one of the most powerful tools for probing the features of surface-modified electrodes [Citation1, Citation2]. It is a rapidly developing electrochemical technique for the characterization of biomaterial functionalized electrodes and biocatalytic transformations at electrode surfaces. The immobilization or biorecognition events of biomaterials, e.g., enzymes, antigens/antibodies or DNA at electrode surfaces change the capacitance and interfacial electron transfer resistance of the electrodes. Impedance spectroscopy allows analysis of interfacial changes originating from biorecognition events at electrode surfaces [Citation3, Citation4].
The reaction rate of β-galactosidase can be monitored by the help of a few alternative methods. Most of these alternative methods are based on spectrophotometric methods. Many studies report that the activity determination of β-galactosidase has been achieved by use of several chromogenic or fluorogenic substances such as ONPG (Ortho-nitrophenyl-β-D-galactopyranoside) and PNPG (para-nitrophenyl-β-D-galactopyranoside) [Citation5–16]. Moreover, such alternative techniques that have been explored include scanning electrochemical microscopy [Citation17], a voltammetric sensor prepared by the immobilization of metallophthalocyanine complexes onto a glassy carbon electrode [Citation18]. Although these technologies have demonstrated successful β-galactosidase activity determination, many require expensive laboratory instrumentation, operator expertise, and may not be easily incorporated into routine analysis.
In this work, a novel aspect of the activity determination of β-galactosidase is presented. A glucose biosensor for β-galactosidase activity determination is reported in this paper, which applies ferricyanide as electron mediator. Glucose oxidase was immobilized and realized the measurement of glucose, which was the product of the reaction of β-galactosidase substrate, lactose in lower voltage. The biosensor (GOD-Glu-pA) was fabricated by crosslinking with glutaraldehyde and then electropolymerization of anilin as a monomer that can provide cheap, simple, and effective immobilization procedure. The activity levels of β-galactosidase can be determined through the measurement of current by ferricyanide mediation seen in the reactions below.
The principle of the activity determination of β-galactosidase was based on the oxidation of Fe[CN]−4 formed by the reaction of glucose oxidase. Enzymatic reaction rate of glucose oxidase in the bioactive layer of the biosensor was increased by the glucose released by β-galactosidase injected to the reaction cell. The reaction rate of glucose oxidase followed by ferricyanide mediation was directly related to the β-galactosidase activity added to the reaction cell as sample or standard solution.
On the basis of cyclic voltammetry and electrochemical impedance investigations, we demonstrated that glucose oxidase was successfully immobilized onto a glassy carbon electrode, and biosensor construction parameters have a marked influence on the modified electrode and play an important role in improving the analytical performance of biosensor. The glucose oxidase immobilized on glassy carbon electrode exhibited excellent electrocatalytical activity to the activity determination of β-galactosidase. The performance of the biosensor was studied in detail.
MATERIALS AND METHODS
Apparatus and Chemicals
Cyclic voltammetry and electrochemical impedance spectroscopy were performed with an AUTOLAB PGSTAT302, Eco Chemie B.V. (Utrecht, The Netherlands) in a conventional three-electrode cell and amperometric experiments were performed with a Metrohm VA 746 Trace Analyzer (Herisau, Switzerland) and VA 747 Stand (Herisau, Switzerland) controlled with a personal computer running the electrochemical software package of Metrohm Trace Analyzer for parameter set-up, data acquisition, and processing. A three electrode configuration was employed in all experiments, with potentials referred to an Ag/AgCl reference electrode. A glassy carbon electrode (Metrohm, Herisau, Switzerland) and a platinum electrode (Metrohm, Herisau, Switzerland) were employed as a working and an auxiliary electrode (2.0 ± 0.1 mm in diameter), respectively. The temperatures of the solution in the electrochemical cell were adjusted by a cryostate, Lauda RE106 (Lavda, Lavda-Königshofen, Germany). The electrolyte solutions were deoxygenated with nitrogen bubbling for 15 min before each voltammetric run and were stirred during the amperometric experiments. Electrochemical impedance measurements were performed in the presence of 0.005 M K3[Fe(CN)6]/K4[Fe(CN)6] (1:1) and 0.1 M KCl mixture as a redox probe in the frequency range between 0.1 Hz and 100000 Hz at the formal potential of the system, Eo = 0.17 V. The amplitude of the alternate voltage was 0.01 V. All experiments were performed at a constant temperature by a thermostated water jacket and under nitrogen atmosphere.
Glucose oxidase (EC 1.1.3.4, from Aspergillus niger), β-galactosidase (EC 3.2.1.23, from Aspergillus oryzae), glutaraldehyde (25%) Albumin from bovine serum (≥96% (agarose gel electrophoresis), lyophilized powder) K3[Fe(CN)6] (99.99+%), K4Fe(CN)6· 3H2O (99.99+%), aniline (≥99.5) lactose, D(+)-glucose, sodium chloride, methanol were obtained from Sigma-Adrich. Sodium hydroxide, potassium dihydrogen phosphate, purchased from Merck.
Preparation of the Biosensor
The surface of the glassy carbon electrode was first polished with 0.05 μm Al2O3 powder and washed ultrasonically in deionized water for 5 minutes. After that, an electrochemical pre-treatment was carried out using multi cycle voltammetric scanning (100X) between (−100) – (−1200) mV at the speed of forward 1 V s−1, and at the speed of backward 10 V s−1 in 0.1 M HNO3 solution. These polishing and pretreatment procedures were repeated before every electrode preparation step. Then a mixture (1:1) of glucose oxidase (90 U) and glutaraldehyde (2.5%) was prepared to be as 20 μL (with pH 7, 0.05 M phosphate buffer solution). In the next step, the mixture was carefully dispersed over the glassy carbon electrode and then dried at 4°C. Polyaniline electropolymerization of biosensors was prepared by electrodeposition from a 0.4 M solution of aniline in phosphate buffer, pH 7, 0.05 M, by holding the potential at 0.6 V for 90 s.
Electrochemical Measurements
Voltammetric measurements of β-galactosidase GOD-Glu-pA was immersed in the thermostated cell (30°C), which contains 20 ml citrate buffer (containing 0.001 M, K3Fe[CN]6) 0.05 M, pH 4.8, and allowed to purge for 15 minutes for removing dissolved oxygen. Then aliquots of standard β-galactosidase solution of different activities were added in the range of 0.01–0.5 U mL−1. At the next step, the reaction cell was stirred at a constant speed for 10 minutes. In this period the hydrolysis reaction of β-galactosidase was realized and this was followed by the reaction of glucose oxidase immobilized at GOD-GLu-pA biosensor. After each addition of β-galactosidase standard the current change is recorded. Then another standard β-galactosidase standard solution of higher concentration is added and so forth. The obtained steady state current peak values are used for the construction of the response curve of the biosensor.
RESULTS AND DISCUSSION
Electrochemical Impedance Spectroscopy and CV Characterization of the Biosensor
As known [Fe(CN)6]4−/3− solution is used as a redox probe in electrochemical impedance spectroscopy (EIS) investigations to give information on impedance changes of the electrode surface in any modification steps of biosensors or sensors [Citation3,Citation4]. In EIS, the semicircle diameter of electrochemical impedance spectrum of a surface signifies the electron transfer resistance Ret. This resistance amplitude controls the electron transfer kinetics of redox probe ([Fe(CN)6]4−/3−) between the electrode and biosensor's layer. Significant differences in the impedance spectra were observed during the preparation of the biosensor. It can be seen from that all impedance spectra show a semicircle. That is to say, the electron-transfer process and some also show a linear portion in the low frequency, corresponding to the diffusion process.
Figure 1. Nyquist plot (Zim vs. Zre) for the Faradaic impedance measurements [In all measurements Fe(CN)63−/4−, 0.005 M + 0.1 M KCl, is used as a redox label in the electrolyte solution: (a) A bare glassy carbon electrode. (b) A glucose oxidase-glutaraldehyde-modified electrode. (c) After electropolymerization of anilin. The frequency range is between 0.1 and 100000 Hz with a signal amplitude of 10 mV. Nyquist plots were obtained at a bias potential of 0.17 V vs Ag/AgCl.
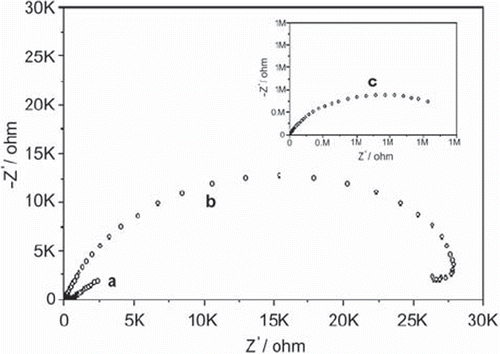
The bare, glassy carbon electrode represented an almost straight line, which was characteristic of a diffusion limited electrochemical process (a). Glucose oxidase-glutaraldehyde layer on the electrode surface generated an important insulating layer on the electrode that acted as a barrier to the interfacial electron transfer (b). The electron transfer resistances, Ret, obtained in these steps were 3.65×102 ohm and 2.87×104 ohm, respectively. Electropolymerization of aniline on the glucose oxidase-glutaraldeyde increased dramatically the electro-transfer resistance, which was 1.38×106 ohm (c). In addition, the insulating characteristics of the layers of biosensor after immobilization were tested by using cyclic voltammetric analysis, as shown in .
Figure 2. Cyclic voltammograms of (a) bare glassy carbon, (b) glassy carbon/glucose oxidase-glutaraldehyde, (c) glassy carbon/glucose oxidase-glutaraldehyde/polyanilin electrodes in 0.1 M KCl solution containing 5 mM Fe(CN)64−/3−. Scan rate: 50 mV s−1.
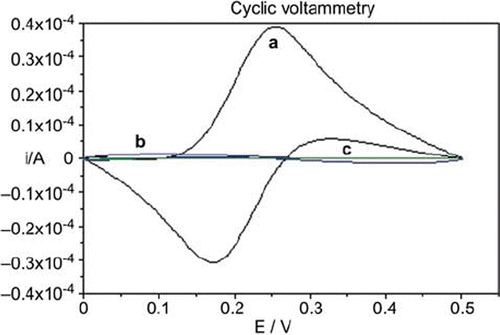
The inner area of the voltammogram shows the glucose oxidase-glutaraldehyde layer and glucose oxidase-glutaraldehyde-polyaniline layers for the redox reaction of Fe(CN)63−/4− (b and c, in ). The cyclic voltammogram of (a) represents the electrochemically reversible shape of the bare glassy carbon electrode for Fe(CN)63−/4−. These data were consistent with the results obtained from electrochemical impedance measurements. Finally, it can be concluded from the data that glucose oxidase was immobilized successively by the immobilization procedure mentioned above.
Optimization Studies of the Biosensor
The fabrication steps of the GOD-Glu-pA biosensor were examined in terms of activity amount of glucose oxidase, glutaraldehyde percentage, aniline concentration, electropolymerization potential of aniline polymerization, electropolymerization period, lactose, and ferricyanide contents of the working buffer, optimum temperature and pH, and the concentration of working buffer. Details are given in the sections below.
The Effect of Glucose Oxidase Activity. In order to determine the optimum amount of glucose oxidase activity for the biosensor construction, altering activity amounts of glucose oxidase (22.5 U, 45 U, 90 U, and 180 U) were used when the other factors were kept constant. The results are given in .
Figure 3. The effect of glucose oxidase activity on the biosensor response [Amounts of glucose activities utilized in biosensors (U): -♦-♦-:45 U, -•-•-:90 U, -▪-▪-:180. Biosensor components: percentages of glutaraldehyde and anilin concentrations were kept constant as 2.5% and 0.4 M, respectively. Electropolymerization potential and polymerization period were 0.6 V and 90 s, respectively. Potential scan conditions: t.puls:40 ms, t.meas:20 ms, U.step:6 mV, scan rate:20 mV s−1. Working buffer was 0.05 M and pH 4.8 citrate solution and contained 1mM ferricyanide as mediator and 0.1 M lactose substrate of β-galactosidase, T = 35°C.]
![Figure 3. The effect of glucose oxidase activity on the biosensor response [Amounts of glucose activities utilized in biosensors (U): -♦-♦-:45 U, -•-•-:90 U, -▪-▪-:180. Biosensor components: percentages of glutaraldehyde and anilin concentrations were kept constant as 2.5% and 0.4 M, respectively. Electropolymerization potential and polymerization period were 0.6 V and 90 s, respectively. Potential scan conditions: t.puls:40 ms, t.meas:20 ms, U.step:6 mV, scan rate:20 mV s−1. Working buffer was 0.05 M and pH 4.8 citrate solution and contained 1mM ferricyanide as mediator and 0.1 M lactose substrate of β-galactosidase, T = 35°C.]](/cms/asset/56d42287-a205-422e-8d9c-8be222188900/ianb19_a_560119_f0003_b.gif)
The most suitable biosensor responses for β-galactosidase were obtained when 90 U glucose oxidase was used. When the activity amount of glucose oxidase was below or above 90 U, the biosensor response decreased. As the activity of glucose oxidase was lower than 90 U, the enzyme molecules in the bioactive layer of the biosensor were insufficient to give such a high response. Moreover, when 22.5 U of glucose oxidase was used to construct the biosensor it could not be possible to form a stable bioactive membrane. On the other hand, when higher activity amounts of glucose oxidase were used, a thicker bioactive membrane was formed in the electrode surface. Consequently, a diffusion barrier was probably caused by such a thicker bioactive membrane.
The Effect of Glutaraldehyde Percentage. To prevent the loss of glucose oxidase, chemical cross-linking is realized between the enzyme and glutaraldehyde. By increasing the concentration of glutaraldehyde (5%), the signals obtained from the biosensor were decreased considerably. Glutaraldehyde can react with amino groups of glucose oxidase, leading to the formation of a bioactive membrane on the glassy carbon electrode. Because of this the thickness of the bioactive membrane was very important in terms of getting good results. As expected, peak currents increased with the decrease in the concentration of glutaraldehyde (2.5%). Improvement of current response of the biosensor with the amount of 2.5% glutaraldehyde was probably related to the formation of the best three-dimensional structure that allowed to diffuse substrate and ferricyanide through the electrode surface. Moreover, 1.25% glutaraldehyde was insufficient to construct a physically stable bioactive membrane. Finally, the optimum glutaraldehyde percentage for the biosensor was 2.5%.
The Optimization of Aniline Concentration. The effect of monomer concentration on the bioactive layer and the results obtained with the biosensor were investigated. For this purpose three different concentrations of anilin (0.2, 0.4 and 0.6 M) were used and the results were compared with each other. β-galactosidase activity calibration graphs were drawn by these data. Linear ranges for β-galactosidase activity determination and R2 values were 0.0235 – 0.376 U, 0.0118 – 0.235 U, 0.0118 – 0.517 U, respectively, and 0.9905, 0.9902, and 0.9784, respectively. In the light of these data, the best biosensor signals were obtained when 0.4 M anilin was used.
The Effects of Electropolymerization Potential and Polymerization Period. In a potentiostatic electropolymerization process, the electropolymerization potential is an important factor that could influence the growing of the polymer. Besides the electropolymerization potential, period for the polymerization could also be important. For this reason, both parameters were optimized for β-galactosidase detection. The results are summarized in .
Table 1. The effects of electropolymerization potential, polymerization peirod, and lactose concentration on the biosensor
In the optimization studies of electropolymerization potential, the results showed that the highest signals were obtained when the potential maintained at 0.6 V. A decrease in the signals occurred at lower potentials than 0.6 V. Similarly, at higher potential than 0.6 V, the signals were also decreased. The decrease at lower potentials was possibly due to insufficient physically stability of the biosensor and the decrease at higher potentials was possibly due to a diffusion barrier, which was caused by a thicker bioactive membrane. In order to optimize the electropolymerization period, the biosensor was prepared by using three different polymerization periods. These results are also given in . As can be seen from the table the time for polianiline plating altered the biosensor signals slightly. As a result, the best electropolymerization potential and the favorable polymerization period were 0.6 V and 90 s, respectively.
The Effects of Concentrations of Lactose as the Substrate and Ferricyanide as a Mediator on the Biosensor. The optimization studies of β-galactosidase biosensor revealed that the most important parameter that influenced the biosensor signals was the concentration of lactose in working buffer. It has been known that if the amount of the enzyme is kept constant and the substrate concentration is then gradually increased, the reaction rate will increase until it reaches a maximum. This is a very common basis of enzymology science. After this point, increases in substrate concentration will not increase the velocity. Consequently it was not surprising to see that the β-galactosidase detection was dramatically affected by the lactose concentration. The results are represented graphically in .
Figure 4. The effect of lactose concentration on the biosensor response [Lactose concentrations tested (mM): -▴-▴-:25, -♦-♦-: 50, -•-•-:100, -▪-▪-:150. Biosensor components: Glucose oxidase activity, percentage of glutaraldehyde and anilin concentrations were kept constant as 90 U, 2.5% and 0.4 M, respectively. Electropolymerization potential and polymerization period were 0.6 V and 90 s, respectively. Potential scan conditions: t.puls: 40 ms, t.meas:20 ms, U.step:6 mV, scan rate:20 mV s−1. Working buffer was 0.05 M and pH 4.8 citrate solution and contained 1mM ferricyanide as mediator 0.1 M lactose substrate of β-galactosidase, T = 35°C.]
![Figure 4. The effect of lactose concentration on the biosensor response [Lactose concentrations tested (mM): -▴-▴-:25, -♦-♦-: 50, -•-•-:100, -▪-▪-:150. Biosensor components: Glucose oxidase activity, percentage of glutaraldehyde and anilin concentrations were kept constant as 90 U, 2.5% and 0.4 M, respectively. Electropolymerization potential and polymerization period were 0.6 V and 90 s, respectively. Potential scan conditions: t.puls: 40 ms, t.meas:20 ms, U.step:6 mV, scan rate:20 mV s−1. Working buffer was 0.05 M and pH 4.8 citrate solution and contained 1mM ferricyanide as mediator 0.1 M lactose substrate of β-galactosidase, T = 35°C.]](/cms/asset/36075d2b-6e74-440e-bd17-5de482a2291e/ianb19_a_560119_f0004_b.gif)
The peak intensity for β-galactosidase increased with the increase in lactose concentration. Moreover, this increase in lactose content gave rise to obtain a wider linear detection range than those of low lactose concentrations. The linear detection ranges obtained for different lactose concentrations are given comparatively in . Further increase in the lactose concentration more than 100 mM did not improve the biosensor signals for β-galactosidase. The best lactose concentration for the β-galactosidase biosensor was accepted as 100 mM.
The effect of mediator concentration on the biosensor response was also studied by varying the concentration of mediator (0.5, 1, and 2 mM). Although the biosensor responses obtained with different mediator concentrations were very close to each other, the best peak intensity was observed with 1 mM ferricyanide. A mediator concentration of 1 mM was selected for all experiments.
Optimum Temperature and Thermal Stability of the Biosensor. The effect of temperature on the biosensor response was investigated in the range of 20 to 45°C. The sensitivity in the range of 30-35°C was almost similar. Typically, when the temperature was below 30°C the biosensor peak density decreased by 50%. When the temperature was higher than 35°C, the response started to decrease considerably. For instance the peak density obtained with 40°C was lower than that of 35°C at a rate of about 14%. However, the difference between the peak currents obtained with 30 and 35°C was just 4%. Consequently, we concluded that it needed to compare operational stabilities of the biosensor at these temperatures. For this purpose calibration graphs for β-galactosidase activity were drawn before and after an incubation period of 5 hours. When the biosensor was incubated at 30°C, the activity of biosensor decreased to approximately 95% for lower activity levels of β-galactosidase and 93.75% for higher activity levels of β-galactosidase. Conversely, if the biosensor was incubated at 35°C, the activity of the biosensor decreased dramatically at the end of this period. The activity of the biosensor was lower by 46% than that of initial activity. Consequently, the appropriate working temperature for the biosensor was accepted as 30°C.
Optimum pH and Buffer Concentration Effect on the Biosensor. It is clearly known that extremely high or low pH values generally result in complete loss of activity for most biosensor systems. The optimum pH will vary greatly from one enzyme to another. In order to study the effect of pH, measurements were carried out in working buffer solutions with altering pH values (50 mM, citrate buffer solutions containing 100 mM lactose). The results revealed that the almost peak-shaped pH profile was obtained. In fact, in our measurement system, our important advantage is that optimum pH values of glucose oxidase and β-galactosidase were very close to each other. If it is conversely the optimum pH values that are very different from each other, it should not be possible to develop and use such a biosensor system. In our experiments, optimum pH value of β-galactosidase probably played a determinative role. Its optimum pH value reported was 4.5 while glucose oxidase could work in the range of pH 4-7. The results agreed with the optimum pHs of both enzymes. For a pH of 4.6, the biosensor signal was about 20% lower than that found in pH 4.8. Moreover, the biosensor response was dramatically decreased at pH values higher than 4.8. This was probably caused from leaving optimum pHs of both enzymes, glucose oxidase and β-galactosidase. As a result, pH 4.8 was the optimum pH for the proposed biosensor.
The effect of the buffer concentration on the biosensor system was also investigated. To evaluate the buffer concentration effect on the biosensor, measurements were made at buffer solutions with different concentrations (25, 50, 100, and 150 mM, pH 4.8, citrate buffers). The highest response was observed when 50 mM citrate buffer solution was used. At lower and higher concentrations, the peak densities were decreased considerably. The peak densities obtained with 25 mM and 100 mM citrate buffers were decreased to 45% and 70%, respectively. The response obtained with 150 mM citrate buffer was lower by 70% than the response obtained with 50 mM citrate buffer.
Characterization Studies of the Biosensor
Linear Range for β-galactosidase Activity. The calibration curve of the biosensor is shown in . The biosensor showed a linear response for an interval of β-galactosidase activity between 0.0118 and 0.47 U mL−1.
Figure 5. β-galactosidase activity graph of the biosensor [Biosensor components: Glucose oxidase activity, percentage of glutaraldehyde and anilin concentrations were kept constant as 90 U, 2.5% and 0.4 M, respectively. Electropolymerization potential and polymerization period were 0.6 V and 90 s, respectively. Potential scan conditions: t.puls:40 ms, t.meas:20 ms, U.step:6 mV, scan rate:20 mV s−1. Working buffer was 0.05 M and pH 4.8 citrate solution and contained 1mM ferricyanide as mediator 0.1 M lactose substrate of β-galactosidase, T = 30°C.]
![Figure 5. β-galactosidase activity graph of the biosensor [Biosensor components: Glucose oxidase activity, percentage of glutaraldehyde and anilin concentrations were kept constant as 90 U, 2.5% and 0.4 M, respectively. Electropolymerization potential and polymerization period were 0.6 V and 90 s, respectively. Potential scan conditions: t.puls:40 ms, t.meas:20 ms, U.step:6 mV, scan rate:20 mV s−1. Working buffer was 0.05 M and pH 4.8 citrate solution and contained 1mM ferricyanide as mediator 0.1 M lactose substrate of β-galactosidase, T = 30°C.]](/cms/asset/e778a04d-ff8d-4eaa-a39d-3295b83005bb/ianb19_a_560119_f0005_b.gif)
As can be seen from the figure, R2 value of the linear plot is quite close to 1. This indicates that the performance of the biosensor is very good. The minimum detectable activity amount of β-galactosidase was estimated to be 0.0118 U mL−1. In the studies, a commercial A.oryzae β-galactosidase was used. Its activity amount was in the level of 9400 U g−1. The detection limit of the biosensor for β-galactosidase could be given as g mL−1 in the reaction cell. The lowest detection limit of the biosensor was 1.25 μg mL−1 of β-galactosidase. This detection limit was perfect for the biosensor.
Operational and Storage Stabilites of the Biosensor. The operational stability of the GOD-Glu-pA electrode was investigated by consecutive measurements of its response to 0.282 U mL−1 β-galactosidase. At the end of the sixteenth measurement the biosensor showed 100% of its initial activity. About 90% initial activity had been retained after 20 measurements. The purpose of storage stability testing was to provide evidence on how the performance of the biosensor based on glucose oxidase for β-galactosidase activity varies with time under the influence of environmental factors such as temperature and humidity. Two different ways were used to investigate correctly the storage stability of the biosensor. In the first way, the biosensors were stored at +4°C for altering periods such as 1, 2, and 3 weeks. The measurements were done at the beginning and end of the storage period. The difference between the signals obtained at first and last was used to understand the storage stability of the biosensor. The biosensor response was constant for the first week. When the biosensor was stored for 2 weeks, the response of the biosensor decreased to about 77%. At the third week of storage period, the biosensor retained about 65% of its initial activity. The storage stability studies of the biosensor clarified that the long-term stability of the biosensor was not as good as expected. An insufficient physically stability of the biosensor was probably resulted in a deficiency in long-term stability of the biosensor. In the second way, as in the first way, the biosensors were stored at +4°C; however, the measurements were done more frequently than the first one. After 3 days’ storage period the biosensor lost no activity. At the end of the seventh and tenth days, the remaining activities of the biosensors were 90% and 80%, respectively. Consequently, this result also supported the idea of the physical in stability of the biosensor mentioned above.
Repeatability and Reproducibility of the Biosensor. The signal changes of the biosensor were investigated when it was consecutive exposed to a 0.282 U mL−1 β-galactosidase standard solution for 10 times. Repeatability of the measurements was very good considering that the correlation coefficient on measurements was 1.8%, and average value and standard deviation were calculated as 0.286 U mL−1 and ± 0.005 U mL−1, respectively. The results showed that the biosensor exhibited a fairly desirable analytical feature of repeatability. The reproducibility of the three same biosensors was also examined. The linear detection ranges of three biosensors were the same as 0.018-0.376 U mL−1. R2 values of the biosensors were 0.9967, 0.9981, and 0.9984.
Sample Analysis. Artificial intestinal juice was prepared to use for sample analysis studies. It contained 10 mM sodium phosphate buffer (pH 7.2), 150 mM sodium chloride, 1 mg mL−1 bovine serum albumin, and 1% (v/v) methanol. A determined unit of β-galactosidase-spiked artificial intestinal juice was analyzed with the help of the biosensor. A reference method was used to compare the results with each other [Citation19]. The results are shown in .
Table 2. β-galactosidase activity determination in artificial intestinal juice by the biosensor and by a spectrophotometric reference method.
Experimental results indicated good recovery was observed. In addition, good agreement between the results of the biosensor presented and the reference method was achieved. These results indicate that the proposed biosensor can be applied successfully for activity determination of β-galactosidase.
CONCLUSION
For β-galactosidase monitoring a new and attractive amperometric biosensor has been presented based on the immobilization of glucose oxidase by glutaraldehyde on the glassy carbon electrode. Although there are some biosensor systems for monitoring of certain hydrolases such as alkaline phosphatase [Citation20–22], the biosensor presented here is the first biosensor for the activity determination of β-galactosidase. A layer of polyaniline was coated on the surface of the glucose oxidase-glutaraldehyde electrode to minimize the loss of GOD in analyzing β-galactosidase and to improve the physical stability of the biosensor. The resulting GOD-Glu-pA biosensor shows very good characteristics, such as a useful determination range, a short response time, a large current density, and high sensitivity. Moreover, the detection limit of the biosensor for β-galactosidase activity was 0.0118 U mL−1. In addition, it meant that the detection limit of β-galactosidase was 1.25 μg mL−1. The enzyme electrode has a reasonable good repeatability and reproducibility. Finally, its applicability to artificial intestinal juice was agreed well with the spectrophotometric reference method.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Cheng, Z.L., Wang, E.K., Yang, X.R. (2001). Capacitive detection of glucose using molecularly imprinted polymers. Biosens Bioelectron, 16: 179–185.
- Jackson, N.M., Hill, M.G. (2001). Electrochemistry at DNA-modified surfaces: new probes for charge transport through the double helix. Curr Opin Chem Biol 5: 209–215.
- Bard, A.J., Faulkner, L.R. (1980). Electrochemical Methods: Fundamentals and Applications. New York: Wiley.
- Stoynov, Z.B., Grafov, B.M., Savova-Stoynova, B.S., Elkin, V.V. (1991). Electrochemical Impedance. Moscow: Nauka.
- Li, S.C., Mazotta, M.Y., Chien, S.F., Li, Y.T. (1970). Isolation and characterization of jack bean β-galactosidase. J Biol Chem 250: 6786–6791.
- Burestedt, E., Nistor, C., Schagerlöf, U., Emneus, J. (2000). An enzyme flow immunoassay that uses β-galactosidase as the label and a cellobiose dehydrogenase biosensor as the label detector. Anal Chem 72: 4171–4177.
- Seeber, F., Boothroyd, J.C. (1996). Escherichia coli beta-galactosidase as an in vitro and in vivo reporter enzyme and stable transfection marker in the intracellular protozoan parasite Toxoplasma gondii. Gene 169: 39–45.
- Manafi, M. (2000). New developments in chromogenic and fluorogenic culture media. Int J Food Microbiol 60:205–218.
- Pelisek, J., Armeanu, S., Nikol, S. (2000). Evaluation of β-galactosidase activity in tissue in the presence of blood. J Vas Res 37: 585–593.
- McGuire, J.B.J., James, T.J., Imber, C.J., Peter, S.D., Friend, P.J., Taylor, R.P. (2002). Optimisation of an enzymatic method for β-galactosidase. Clin Chim Acta 326: 123–129.
- Mássen, M., Liu, Z., Haruyama, T., Kobatake, E., Ikariyama, Y., Aizawa, M. (1995). Immunosensing with amperometric detection, using galactosidase as label and p-aminophenyl-β-D-galactopyranoside as substrate. Anal Chim Acta 304: 353–359.
- Pérez, F., Tryland, I., Macsini, M., Fiksdal, L. (2001). Rapid detection of Escherichia coli in water by a culture-based amperometric method. Anal Chim Acta 427: 149–154.
- Madoz, J., Medrano, F.J., Garcia, J.L., Fernandez, V.M. (1997). Functionalization of gold surfaces for specific and reversible attachment of a fused beta-galactosidase and choline-receptor protein. J Am Chem Soc 119: 1043–1051.
- Scott, D.L., Ramanathan, S., Shi, W., Rosen, B.P., Daunert, S. (1997). Genetically engineered bacteria: Electrochemical sensing systems for antimonite and arsenite. Anal Chem 69:16–20.
- Zinselmeyer, B.H., Beggbie, N., Uchegbu, I.F., Schätzlein, A.G. (2003). Quantification of β-galactosidase activity after non-viral transfection in vivo. J Cont Rel 91: 201–208.
- Gary, R.K., Kindell, S.M. (2005). Quantitative assay of senescence-associated β-galactosidase activity in mammalian cell extracts. Anal Biochem 343: 329–334.
- Zhao, C., Sinha, J.K., Wijayawardhana, C.A., Wittstock, G. (2004). Monitoring β-galactosidase activity by means of scanning electrochemical microscopy. J Electroanal Chem 561: 83–91.
- Bahl, O.P., Agrawal, K.M.L. (1969). Glycosidases of aspergillus niger. I. Purification and characterization of α- and β-galactosidase and β-N-acetylglucosaminidase. J Biol Chem 244: 2970–2978.
- Wutor, V.C., Togo, C.A., Limson, J.L., Pletschke, B.I. (2007). A novel biosensor for the detection and monitoring of [Beta]-d-galactosidase of faecal origin in water. Enz Microbial Tech 40:1512–1517.
- Díaz, A.N., Sánchez, F.G., Ramos, M.C., Torrijas, M.C. (2002). Horseradish peroxidase sol–gel immobilized for chemiluminescence measurements of alkaline-phosphatase activity. Sens Actuat B 82:176–179.
- Ito, S., Yamazaki, S., Kano, K., Ikeda, T. (2000). Highly sensitive electrochemical detection of alkaline phosphatase. Anal Chim Acta 424:57–63.
- Serra, B., Morales, M.D., Reviejo, A.J., Hall, E.H., Pingarrón, J.M. (2005). Rapid and highly sensitive electrochemical determination of alkaline phosphatase using a composite tyrosinase biosensor. Anal Biochem 336: 289–294.