Abstract
Abstract: Targeted and simultaneous delivery VEGF165b and IFN alpha in anti-angiogenic and other applications could offer several advantages. For this a system was design using artificial cell alginate-poly-L-lysine- alginate (APA) microcapsules. Result confirms the ability of this system for simultaneous production of these proteins for 28-days. The IFN alpha on a 3 days period increased from 8 ± 0.36 μg/ml at day 10 to 27 ± 2.4 μg/ml at day 16 and then dropped to 6.5 ± 0.5 μg/ml. The VEGF165b on a 3 days period increased from 2.7 ± 0.7 μg/ml at day 10 to 6.9 ± 1 μg/ml at day 16.
Introduction
Artificial cell microencapsulation therapy has offered the potential treatment for various diseases and presented a preferable system for controlled and continuous delivery of bioactive agents [Citation1–3]. Encapsulation of the living cells provides the possibility of implanting the cells and protecting them from rejection after implantation. The membrane of the microcapsules is a permselective barrier that eliminates the effect of host immune mediators responsible for graft rejection and presents free access of nutrients for encapsulated cells [Citation4]. Thus the encapsulated cells remain viable for an extended time after implantation and provide continuous source of desired products [Citation5–7]. This system can be applied for delivering angiogenesis inhibitors to the target site for tumor suppression.
One of the growing areas in research is the possibility of starving tumor cells from nutrients, which leads to shrinking the tumor and preventing the metastasis [Citation8–10]. The growing tumors constantly stimulate the creation of the new vasculature to access the required nutrients by expression of angiogenic factors such as bFGF, aFGF, vascular permeability growth factors (VPF) and vascular endothelial growth factor (VEGF) [Citation11–13]. Angiogenesis inhibitors that block tumor's blood supply have been considered as cancer fighters. In this research two angiogenesis inhibitors VEGF165b and IFNα were studied. VEGF165b is an inhibitory splice variant of VEGF [Citation14]. It is expressed in normal cells and tissues and is circulating in human plasma. VEGF165b binds VEGF receptor 2, but unlike other VEGF isoforms rather than stimulating endothelial cell proliferation or migration, it inhibits the growth of new blood vessels required for tumor growth [Citation15–17].
IFNα is the first known antiangiogenesis factor [Citation11]. This multifunctional cytokine [Citation18] restrains angiogenesis by the ability to down-regulate expression of tumor induced angiogenic factors such as bFGF, VEGF and MMP-9. IFNα inhibits cell migration in a dose dependent and reversible manner 19,20]. Research on IFNα demonstrated that for the optimum efficacy of an IFNα-based antiangiogenic therapy, its continuous and long-term delivery is necessary. Therefore in order to provide a continuous source of protein, cell lines have been engineered to release the desired protein stably. As mentioned above, to implant the engineered cell lines, they are enclosed in immunoprotective microcapsules to maintain their viability and their ability to secret the product.
In this study, two cell lines, VEGF165b producing Human Embryonic Kidney 293 (HEK293) cells and IFNα producing HEK293 cells were co-encapsulated in APA microcapsules and their viability and their ability to produce proteins were analyzed. Co-encapsulation can offer the advantage of being able to produce both antiangiogenic proteins simultaneously.
Materials and methods
Materials
Freestyle or Freestyle-F17 medium, Blasticidin, NuPAGE 4X sample buffer and NuPAGE 4-12% Bis-Tris gel were obtained from Invitrogen. Pluronic F68 was purchased from Sigma-Aldrich. Geniticin was obtained from Wisent. Alginate (low viscosity) and poly-L-lysine were obtained from Sigma-Aldrich. MTS colorimetric assay kit was purchased from Promega. Antibodies, rabbit anti-VEGF from Santa cruise and rabbit anti-human IFNα from PBL InterferonSource were purchased. BM Chemiluminescent Blotting kit was obtained from Roche. Human VEGF Quantikine ELISA kit from R&D systems and Human Interferon alpha ELISA kit from PBL InterferonSource were purchased.
Methods
Cell line and culture condition. The human embryonic kidney 293 (HEK293) cell line, stably expressing Epstein-Barr virus Nuclear Antigen-1 (clone 6E) has been developed at Biotechnology Research Institute of Montreal. VEGF165b producing HEK293 cells (clone F10) and IFNα producing HEK293 cells (clone D9) were derived from HEK293 cells. They were grown as a suspension culture in FreeStyle or Freestyle-F17 (F17; Invitrogen) medium, a serum- and protein-free medium, supplemented with 0.1% pluronic F-68, 50 μg/ml Geneticin and 0.5 μg/ml Blasticidin. Cells were grown agitated at 110 rpm at 37°C in a humidified, 5% CO2 chamber and routinely passed in 125-ml Erlenmeyer flasks containing 20 ml of culture medium.
Co-encapsulation of transfected cells in APA (Alginate-poly-l-lysine-alginate) microcapsules. The VEGF165b producing HEK293 cells (clone F10) and IFNα producing HEK293 cells (clone D9) were co-encapsulated as described previously with slight modification [Citation21,Citation22]. Cells were centrifuged at 1000 g for 10 min. The supernatant was removed and the cells were mixed and resuspended in a sterile filtered 1.65% (w/v) alginate (obtained from Sigma-Aldrich, low viscosity) solution at a concentration of 1 × 106 cells/ml. Alginate solution was made in 0.85% (w/v) saline. The mixture of cells and alginate solution was loaded in a 60 ml syringe and extruded through an Inotech Encapsulator IER-20 (Inotech Biosystems International, Rockville, MD) with a 300 μm nozzle at a frequency of 1052 Hz and a voltage of 1.0 kV. Alginate droplets were collected in 1.5% CaCl2 solution and stirred for 30 minutes to harden the gel. To form the microcapsules membrane, Ca-alginate beads were incubated in a 0.1% (w/v) poly-L-lysine (Sigma, MW 27400) solution for 15 minutes followed by a 10-minutes incubation in a 0.1% (w/v) alginate solution. All solutions were made in 0.85% (w/v) saline. The final microcapsules were stored at 37°C in a humidified, 5% CO2 chamber in culture media for further analysis.
Determining the viability and metabolic activity of encapsulated cells. The metabolic activity and cell proliferation of encapsulated cells was determined using a MTS colorimetric assay. The assay is based on the ability of dehydrogenase enzymes found in metabolically active cells to convert MTS into a formazan product that is soluble in culture medium. The quantity of formazan product as measured by the amount of 490 nm absorbance is directly proportional to the number of living cells in culture.
The assay was performed according to previously described procedures 23,24] with slight modifications. Every three days, approximately 50 ± 5 microcapsules were transferred to a 96-well plate and incubated with 100 μl media and 20 μl MTS/PMS solution at 37°C for 4 hours. The optical density was read and recorded at 490 nm using a plate reader. The cells were quantified using a calibration curve correlating cell number with absorbance.
Western Blot analysis of protein productivity of encapsulated cells. To verify the protein production of microencapsulated VEGF165b producing HEK293 cells and IFNα producing HEK293 cells, encapsulated cells were suspended in F17 medium and incubated at 37°C in a humidified, 5% CO2 chamber. The samples were taken every three days and analyzed by western blot for the presence of VEGF165b and IFNα. The harvested medium from encapsulated cells were diluted in NuPAGE 4X sample buffer containing 50 mM DTT and then heated at 70°C for 10 min. Protein separation was performed on NuPAGE 4-12% Bis-Tris gel using MES running buffer for 40 min at 200 V (voltage). Western blots were performed by transferring proteins to nitrocellulose membrane using Tris-glycine buffer for 1 hour at 300 mA (mili amperage). The membrane was then incubated with a rabbit anti-VEGF or rabbit anti-IFNα diluted 1:500 for 1 hour followed by incubation with an anti-rabbit horseradish peroxidase (1:5000) for 1 hour. BM Chemiluminescent Blotting kit was used to evaluate the blots.
VEGF165b and IFNα quantification by ELISA. ELISA was used to measure the quantity of VEGF165b and IFNα produced by encapsulated cells. The quantity of VEGF165b was determined using Human VEGF Quantikine ELISA kit. According to the protocol, assay diluent was added to each well of the plate followed by adding samples, standards and blank. The plate was covered and incubated for 2 hours at room temperature. After 2 hours each well was aspirated and washed three times with wash buffer. VEGF conjugate was then added to each well; the plate was covered and incubated at room temperature. After 2 hours incubation, washing step was repeated as mentioned above. Substrate solution was then added to all wells. The plate was protected from light and incubated for 20 minutes at room temperature. Then stop solution was added and mixed thoroughly to uniform the color. The optical density was determined within 30 minutes using a microplate reader at 450 nm.
To determine the quantity of IFNα, Human Interferon alpha ELISA kit was used. According to the protocol, samples, blanks and standards were added to each well; the plate was covered and incubated for 1 hour. After 1 hour the contents were emptied and the wells were washed with diluted wash buffer for one time. Then HRP was added to all wells; the plate was covered and incubated for 1 hour. After 1 hour the wells were emptied and washed four times with wash buffer. Then TMB substrate solution was added to each well. The plate was incubated in a dark place. After 15 minutes incubation stop solution was added to each well and the absorbance was determined within 5 minutes at 450 nm using a microplate reader.
Results
Morphological studies and Viability of encapsulated cells
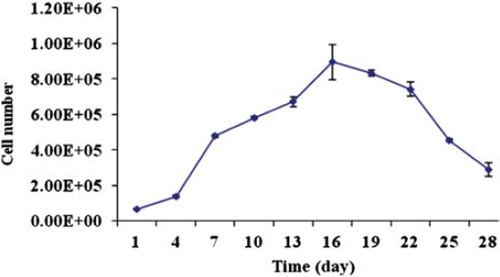
APA microcapsules were prepared and the microcapsules were evaluated. Results showed that microcapsules with a diameter of 500 ± 50 μm could be prepared (). Results also showed that the cells were evenly distributed within the microcapsules. Viability of encapsulated cells was evaluated using MTS assay. Results showed that the encapsulated VEGF165b producing HEK293 cells and IFNα producing HEK293 cells continued to grow from day first to day 16. Then the viability of encapsulated cells decreased slowly until day 28 of the experiment ().
Figure 1. Photomicrograph of alginate-poly-L-lysine-alginate microcapsules (A) APA microcapsules without cells (B) APA microcapsules containing co-encapsulated engineered cells producing IFNα and engineered cells producing VEGF165b.
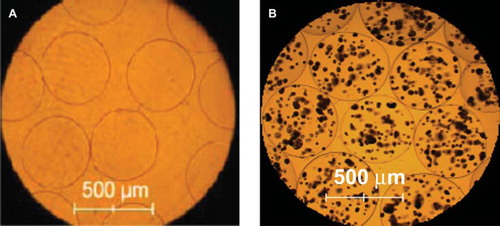
Figure 2. The number of viable co-microencapsulated cells determined by MTS assay. The capsules contain VEGF165b producing HEK293 cells and IFNα producing HEK293 cells.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blot were performed to analyze VEGF165b and IFNα produced by encapsulated cells. The production of IFNα was detected on the blot from day 4 to day 28. Results showed that IFNα was produced similarly between days 10 and 25. The production of protein continued and it was detectable on the blot until day 28 ().
Figure 3. Western blot analysis of human IFNα and human VEGF165b produced by co-encapsulated cells. (A) Production of IFNα (B) production of VEGF165b at various time periods
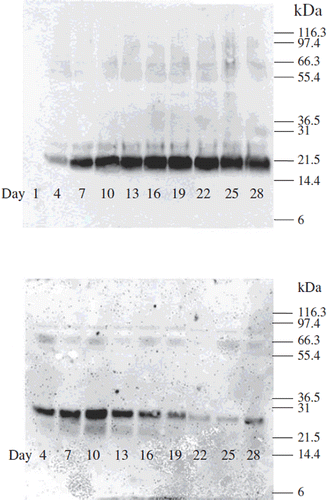
Results from western blot analysis for the production of VEGF165b showed that VEGF165b production was detected from day 4 to day 28. The highest production was observed between days 7 to 19 in which the highest amount was detected at day 10. VEGF165b production decreased clearly after day 19 (). Western blot analysis also showed that the production of IFNα and VEGF165b didn't follow the same pattern. VEGF165b was produced in lower quantity with the highest production at day 10 however IFNα was produced more with the pick of production at day 16 which was compatible with the cell growth curve.
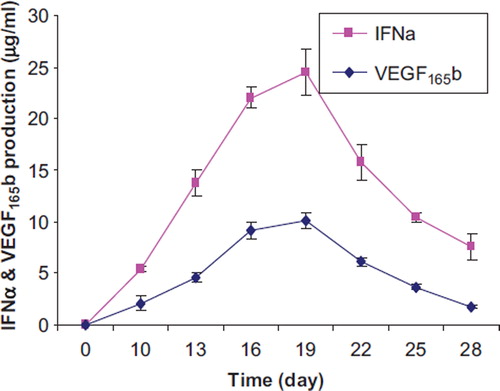
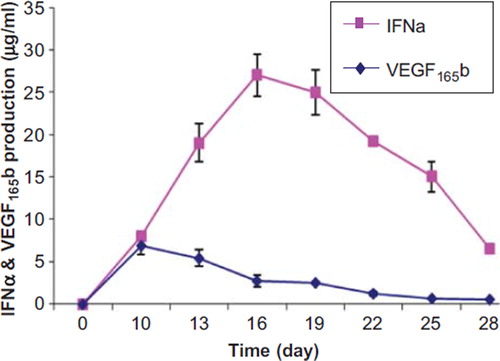
We also measured the quantity of IFNα and VEGF165b by ELISA. Results showed that the highest quantity of IFNα was measured at 27 ± 2.4 μg/ml and 25 ± 2.6 μg/ml between days 16 and 19. The quantity of IFNα decreased slowly to 6.5 ± 0.5 μg/ml for the last day of the experiment. At day 10 the quantity of VEGF165b was measured 6.9 ± 1 μg/ml and then the values decreased to 0.54 ± 0.06 μg/ml for the last day of the experiment ().
Figure 4. The quantity of secreted IFNα and VEGF165b from co-encapsulated cells determined by ELISA.
We also compared the quantity of produced proteins by encapsulated cells with non-encapsulated cells. Non-encapsulated cells followed similar pattern in production of proteins. The highest quantity of produced IFNα by free cells was measured at 24.5 ± 2.2 μg/ml for day 19, then the production decreased to 7.6 ± 1.2 μg/ml for the last day of the experiment. Non-encapsulated cells produced the highest quantity of VEGF165b between days 16 and 19. The value dropped from 10.11 ± 0.2 μg/ml at day 19 to 1.8 ± 0.14 μg/ml at day 28 ().
Discussion
Delivering therapeutic proteins in a continuous low dose from a source of encapsulated living cells has many advantageous over the conventional therapy of direct administration of therapeutic proteins. Once recombinant cells are designed to stably produce proteins and properly encapsulated, they can be implanted in any host without requiring any genomic modification in recipient. The feasibility of this approach has been reported for the production of local and sustained delivery of proteins [Citation25], for example sustained delivery of human growth hormone by implanting the encapsulated engineered fibroblasts in mice [Citation26], continuous delivery of interleukin 12 from microencapsulated engineered cell for colon cancer therapy [Citation27] and tumor suppression using microencapsulated engineered cells producing tumor necrosis factor-α can be mentioned. Also this approach is acknowledged as an attractive antiangiogenic tumor therapy and offers a continuous source of angiogenesis inhibitors, which is a valuable treatment in many solid tumors. Development of this strategy has made the possibility of delivering recombinant proteins to tumors by implanting encapsulated producer cells. Local or systemic delivery of endostatin [29–35] and angiostatin [36–38] from microencapsulated recombinant cells for antiangiogenic cancer therapy have been reported by many research groups, indicating the importance of this approach for an alternative or an adjuvant in cancer therapy. In this work we also studied the release of two angiogenesis inhibitors from encapsulated cells. VEGF165b is an inhibitory vascular endothelial growth factor splice variant; it does not stimulate endothelial cell proliferation or migration and appeared to be down-regulated in some tumors [Citation17]. This angiogenesis inhibitor has potential key role in blocking angiogenesis due to triggering VEGF. Among growth factors regulating angiogenesis, VEGF is considered the most important factor. The expression of VEGF is elevated in the majority of human tumors and plays a central role in mediating angiogenesis in tumor growth and metastasis [Citation39–41]. Although blocking VEGF is very important in antiangiogenesis treatment, it does not appear sufficient for tumor regression by itself, since angiogenesis is a complex process and involves different regulatory factors [Citation13]. To enhance the inhibitory effects of VEGF165b and prevent the likelihood of finding a compensatory pathway by tumors to survive, we studied the combination therapy with IFNα. IFNα has shown successful angiogenesis inhibitory effects [Citation42,Citation43] with the mechanism of down-regulating tumor –induced angiogenic factors including VEGF, bFGF and MMP-9 [Citation44,Citation45]. In this study, the combination of two stable clones producing VEGF165b and IFNα was applied to perform co-encapsulation so that the amount of produced protein released from microcapsules can be adjusted by using different ratio of producing cells.
The analysis of protein production showed that both proteins have been successfully produced as detected on the blot and measured by ELISA. However the production of IFNα was higher than VEGF165b, which indicated that there might be a shift in production of IFNα vs. VEGF165b. Therefore to potentiate the angiogenesis inhibition of VEGF165b, various ratios of the two cell lines could be used to obtain the desired amount of each protein. This system offers several advantageous over the single production of each protein such as being able to titer both proteins by using various ratios of the two cell lines, cost and ease of manipulation since there is one single system for production of two proteins. However to block the angiogenesis in tumors in order to convert a metastatic tumor to a chronic dormant disease, the synergistic effects of inhibitors in combination therapy, the accurate dosage of each inhibitor and the duration of treatment have to be evaluated in animal models.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Reference
- Orive, G., Hernandez, R.M., Gascon, A.R., Calafiore, R., Chang, T.M., de Vos, P., Hortelano, G., Hunkeler, D., Lacik, I., Shapiro, A.M., and Pedraz, J.L. (2003). Cell encapsulation: promise and progress. Nat. Med., 9:104–107.
- Chang T.M, (2000). Artificial cell biotechnology for medical applications. Blood Purif., 18: 91–96.
- De Vos P., Faas M.M., Strand, B., and Calafiore, R. (2006). Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials, 27: 5603–5617.
- Lanza R.P, Hayes J.L, andChick W.L, (1996). Encapsulated cell technology. Nat. Biotechnol., 14: 1107–1111.
- Lahooti, S. and Sefton M.V, (2000). Effect of an immobilization matrix and capsule membrane permeability on the viability of encapsulated HEK cells. Biomaterials, 21: 987–995.
- Orive, G., Hernandez R.M, Gascon A.R, Igartua, M., Rojas, A., andPedraz J.L, (2001). Microencapsulation of an anti-VE-cadherin antibody secreting 1B5 hybridoma cells. Biotechnol. Bioeng., 76: 285–294.
- Chang P.L, Van Raamsdonk J.M., Hortelano, G., Barsoum S.C, MacDonald N.C., andStockley T.L, (1999). The in vivo delivery of heterologous proteins by microencapsulated recombinant cells. Trends Biotechnol., 17: 78–83.
- O'Reilly M.S, Boehm, T., Shing, Y., Fukai, N., Vasios, G., Lane W.S, Flynn, E., Birkhead J.R, Olsen B.R, andFolkman, J. (1997). Endostatin: An endogenous inhibitor of angiogenesis and tumor growth. Cell, 88: 277–285.
- Noonan D.M, Benelli, R., andAlbini, A. (2007). Angiogenesis and cancer prevention: A vision. Recent Results Cancer Res., 174: 219–224.
- Folkman, J. (2004). Endogenous angiogenesis inhibitors. APMIS, 112: 496–507.
- Folkman, J. (2006). Angiogenesis. Annu. Rev. Med., 57: 1–18.
- Verheul H.M, Voest E.E, and Schlingemann R.O, (2004). Are tumours angiogenesis-dependent? J. Pathol., 202: 5–13.
- Lamalice, L., Le Boeuf, F., andHuot, J. (2007). Endothelial cell migration during angiogenesis. Circ. Res., 100: 782–794.
- Bates D.O, Cui T.G, Doughty J.M, Winkler, M., Sugiono, M., Shields J.D, Peat, D., Gillatt, D., andHarper S.J, (2002). VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res., 62: 4123–4131.
- Bates D.O, andHarper S.J, (2005). Therapeutic potential of inhibitory VEGF splice variants. Future. Oncol., 1: 467–473.
- Konopatskaya, O., Churchill A.J, Harper S.J, Bates D.O, andGardiner T.A, (2006). VEGF165b, an endogenous C-terminal splice variant of VEGF, inhibits retinal neovascularization in mice. Mol. Vis., 12: 626–632.
- Woolard, J., Wang W.Y, Bevan H.S., Qiu, Y., Morbidelli, L., Pritchard-Jones R.O, Cui T.G, Sugiono, M., Waine, E., Perrin, R., Foster, R., Digby-Bell, J., Shields J.D, Whittles C.E, Mushens R.E, Gillatt D.A., Ziche, M., Harper S.J., andBates D.O, (2004). VEGF165b, an inhibitory vascular endothelial growth factor splice variant: Mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res., 64: 7822–7835.
- Sidky, Y. A. andBorden E.C, (1987). Inhibition of angiogenesis by interferons: effects on tumor- and lymphocyte-induced vascular responses. Cancer Res., 47: 5155–5161.
- Rosewicz, S., Detjen, K., Scholz, A., andvon Marschall, Z. (2004). Interferon-alpha: Regulatory effects on cell cycle and angiogenesis. Neuroendocrinology, 80 Suppl 1: 85–93.
- Streck C.J, Ng C.Y., Zhang, Y., Zhou, J., Nathwani A.C, andDavidoff A.M, (2005). Interferon-mediated anti-angiogenic therapy for neuroblastoma. Cancer Lett., 228: 163–170.
- Peirone, M., Ross C.J, Hortelano, G., Brash J.L, andChang, P. L. (1998). Encapsulation of various recombinant mammalian cell types in different alginate microcapsules. J. Biomed. Mater. Res., 42: 587–596.
- Ouyang, W., Chen, H., Jones M.L, Metz, T., Haque, T., Martoni, C., andPrakash, S. (2004). Artificial cell microcapsule for oral delivery of live bacterial cells for therapy: Design, preparation, and in-vitro characterization. J. Pharm. Pharm. Sci., 7: 315–324.
- Bunger C.M, Jahnke, A., Stange, J., De Vos, P., andHopt U.T, (2002). MTS colorimetric assay in combination with a live-dead assay for testing encapsulated L929 fibroblasts in alginate poly-L-lysine microcapsules in vitro. Artif. Organs, 26: 111–116.
- Stensvaag, V., Furmanek, T., Lonning, K., Terzis A.J, Bjerkvig, R., andVisted, T. (2004). Cryopreservation of alginate-encapsulated recombinant cells for antiangiogenic therapy. Cell Transplant., 13: 35–44.
- Wen, J., Vargas A.G, Ofosu F.A, andHortelano, G. (2006). Sustained and therapeutic levels of human factor IX in hemophilia B mice implanted with microcapsules: Key role of encapsulated cells. J. Gene Med., 8: 362–369.
- Tai I.T, andSun A.M, (1993). Microencapsulation of recombinant cells: A new delivery system for gene therapy. FASEB J., 7: 1061–1069.
- Zheng, S., Xiao Z.X, Pan Y.L, Han M.Y, andDong, Q. (2003). Continuous release of interleukin 12 from microencapsulated engineered cells for colon cancer therapy. World J. Gastroenterol., 9: 951–955.
- Hao, S., Su, L., Guo, X., Moyana, T., andXiang, J. (2005). A novel approach to tumor suppression using microencapsulated engineered J558/TNF-alpha cells. Exp. Oncol., 27: 56–60.
- Bjerkvig, R., Read T.A., Vajkoczy, P., Aebischer, P., Pralong, W., Platt, S., Melvik J.E., Hagen, A., andDornish, M. (2003). Cell therapy using encapsulated cells producing endostatin. Acta Neurochir. Suppl, 88: 137–141.
- Joki, T., Machluf, M., Atala, A., Zhu, J., Seyfried N.T, Dunn I.F., Abe, T., Carroll R.S., andBlack P.M, (2001). Continuous release of endostatin from microencapsulated engineered cells for tumor therapy. Nat. Biotechnol., 19: 35–39.
- Read T.A., Farhadi, M., Bjerkvig, R., Olsen B.R, Rokstad A.M, Huszthy P.C., andVajkoczy, P. (2001). Intravital microscopy reveals novel antivascular and antitumor effects of endostatin delivered locally by alginate-encapsulated cells. Cancer Res., 61: 6830–6837.
- Read T.A, Sorensen D.R., Mahesparan, R., Enger P.O., Timpl, R., Olsen B.R., Hjelstuen M.H., Haraldseth, O., andBjerkvig, R. (2001). Local endostatin treatment of gliomas administered by microencapsulated producer cells. Nat. Biotechnol., 19: 29–34.
- Teng, H., Zhang, Y., Wang, W., Ma, X., andFei, J. (2007). Inhibition of tumor growth in mice by endostatin derived from abdominal transplanted encapsulated cells. Acta Biochim. Biophys. Sin.(Shanghai), 39: 278–284.
- Zhang, Y., Wang, W., Zhou, J., Yu, W., Zhang, X., Guo, X., andMa, X. (2007). Tumor anti-angiogenic gene therapy with microencapsulated recombinant CHO Cells. Ann. Biomed. Eng., 35: 605–614.
- Zhang, Y., Wang, W., Xie, Y., Yu, W., Teng, H., Liu, X., Zhang, X., Guo, X., Fei, J., andMa, X. (2007). In vivo culture of encapsulated endostatin-secreting Chinese hamster ovary cells for systemic tumor inhibition. Hum. Gene Ther., 18: 474–481.
- Cirone, P., Bourgeois J.M., Shen, F., andChang P.L, (2004). Combined immunotherapy and antiangiogenic therapy of cancer with microencapsulated cells. Hum. Gene Ther., 15: 945–959.
- Cirone, P., Shen, F., andChang, P. L. (2005). A multiprong approach to cancer gene therapy by coencapsulated cells. Cancer Gene Ther., 12: 369–380.
- Visted, T., Furmanek, T., Sakariassen, P., Foegler W.B., Sim, K., Westphal, H., Bjerkvig, R., andLund-Johansen, M. (2003). Prospects for delivery of recombinant angiostatin by cell-encapsulation therapy. Hum. Gene Ther., 14: 1429–1440.
- Ferrara, N. (2004). Vascular endothelial growth factor as a target for anticancer therapy. Oncologist., 9 Suppl 1: 2–10.
- Ferrara, N. (2005). VEGF as a therapeutic target in cancer. Oncology, 69 Suppl 3: 11–16.
- Frumovitz, M. andSood, A. K. (2007). Vascular endothelial growth factor (VEGF) pathway as a therapeutic target in gynecologic malignancies. Gynecol. Oncol., 104: 768–778.
- Marler J.J, Rubin J.B., Trede N.S., Connors, S., Grier, H., Upton, J., Mulliken J.B., andFolkman, J. (2002). Successful antiangiogenic therapy of giant cell angioblastoma with interferon alfa 2b: Report of 2 cases. Pediatrics, 109: E37.
- Singh R.K., Gutman, M., Bucana C.D., Sanchez, R., Llansa, N., andFidler I.J, (1995). Interferons alpha and beta down-regulate the expression of basic fibroblast growth factor in human carcinomas. Proc. Natl. Acad. Sci. U.S.A, 92: 4562–4566.
- Slaton J.W., Perrotte, P., Inoue, K., Dinney C.P., andFidler, I. J. (1999). Interferon-alpha-mediated down-regulation of angiogenesis-related genes and therapy of bladder cancer are dependent on optimization of biological dose and schedule. Clin. Cancer Res., 5: 2726–2734.
- Wang, L., Wu W.Z, Sun H.C, Wu X.F., Qin L.X., Liu Y.K., Liu K.D., andTang Z.Y, (2003). Mechanism of interferon alpha on inhibition of metastasis and angiogenesis of hepatocellular carcinoma after curative resection in nude mice. J. Gastrointest. Surg., 7: 587–594.