Abstract
Abstract: We have previously reported that derivatization of hemoglobin with periodate-modified sugar derivatives such as oxidized adenosine triphosphate (oATP) leads to an increase in prooxidant reactivity at the heme. Here, we report that copolymerization of hemoglobin with serum albumin alleviates this problem completely, to the extent where the copolymer even has a slightly lower autooxidation rate compared to native hemoglobin. A similar, although not as potent, effect is obtained when hemoglobin is derivatized with oATP in the presence of small-molecule antioxidants instead of albumin.
INTRODUCTION
Blood substitutes based on acellular hemoglobin are often marked by increased pro-oxidant reactivity [Citation1–11]. The first product submitted for clinical trials featured an increased autooxidation rate, among the very few signs at the in vitro stage that may have predicted the eventual failure in clinical trials [Citation3]. Other derivatization procedures also result in hemoglobin autooxidation, so that antioxidants or anaerobic conditions are sometimes employed to alleviate this problem [Citation4, Citation9].
Other parameters, such as dioxygen binding affinity and molecule size, have also been shown to be important for designing a successful blood substitute; strategies for tackling these issues have included polymerization, encapsulation, or derivatization of the protein surface with biocompatible polymers [Citation12–14].
We have recently reported polymerization of hemoglobin with a range of periodate-oxidized hydroxyl-containing compounds, including adenosine triphosphate (ATP), starch, and polyethylene glycol (PEG) [Citation5]. A common feature of all these products was an autooxidation rate increased by 120-190%, and oxidation of the hemoglobin during the derivatization protocol [Citation5]. Here, we report that co-polymerization of hemoglobin with albumin not only alleviates such problems, but also lowers the autooxidation rate to a level slightly below that of native hemoglobin. Small-molecule antioxidants display, to a certain degree, similar effects. These data add to a growing body of evidence according to which use of antioxidants and antioxidant enzymes is beneficial in potential blood substitute preparations [Citation15–17].
MATERIALS AND METHODS
Hemoglobin was purified following a general protocol of Antonini and Brunori [Citation18], which involves only centrifugation, salt precipitation with ammonium sulfate and dialysis – and thus results in what may be referred to as stroma-free hemoglobin, which retains catalase and superoxide dismutase enzymes originating from the erythrocyte. The presence of such antioxidant enzymes is likely to be advantageous for blood substitute purposes [Citation15–17]. Bovine serum albumin (BSA, fraction V, from Sigma, Germany) was used as provided without further purification. Proteins were manipulated in phosphate buffer saline (PBS) unless otherwise mentioned. Hemoglobin concentrations in the text are given per heme rather than per tetramer. Adenosine triphosphate (ATP, Merck, Germany) and hydrogen peroxide (30%, Sigma-Aldrich, Germany) were used as received.
For ATP oxidation, the protocol followed indications given by Palmer and co-workers [Citation8]: 50 mM solutions of ATP were prepared in 18.1 MΩ deionized water. The solution was oxidized with sodium periodate (NaIO4) for 1 hour at room temperature in order to ring-open the 1,2-diols to yield dialdehydes. The product of ATP oxidation via this protocol is designated as oATP. For polymerization of Hb with oATP, 1 mM Hb was reacted with 10 mM oATP. The reaction was performed under stirring at 4°C; a twofold excess of NaBH4 was then added to each reaction vessel for 30 min to quench the reaction. The product was dialyzed in 50 mM Tris buffer with 150 mM NaCl (pH 7.4) to remove excess NaBH4 and unreacted cross-linker. The protein was then chromatographed on a Sephacryl S-300 size exclusion column (GE Healthcare, Sweden) with a mobile phase of 150 mM NaCl, 20 mM Tris buffer at pH 7.4. The derivatized forms of hemoglobin were, where required, separated using a HiTrap SP HP anion exchange column (GE Healthcare) controlled by a FPLC system. Using 20 mM Tris-Cl pH 7.4 as mobile phase, the derivatized Hb was bound to the stationary phase of the column, whereas the unmodified Hb was not. The modified Hb was then eluted with Tris buffer pH 7.4 containing 200-1000 mM NaCl. The separated protein fractions were further analyzed by 15 % SDS-PAGE and gel filtration size exclusion chromatography on a Superdex 200 5/150 GL column (GE Healthcare); molecular weights were determined based on a calibration curve employing a molecular weight standard kit (Sigma-Aldrich) containing carbonic anhydrase (29 kDa), bovine serum albumin, (BSA, 66 kDa), alcohol dehydrogenase (150 kDa), amylase (200 kDa), apoferritin (443 kDa), thyroglobulin (669 kda) and blue dextran (void volume marker).
Additionally, the following substances were added to the polymerization reaction mixture as potential antioxidants with protective effects against hemoglobin autooxidation: BSA (bovine serum albumin), ascorbic acid, sodium thiosulfate, and glucose. The antioxidant was added to hemoglobin before the oATP at the following concentrations: BSA - 1μM, 10μM, 100μM, 500μM, 1mM, 2mM; ascorbate - 10 mM; thiosulfate - 15mM; glucose - 2mM. For calculating the amounts of oxy, met, and hemichrome forms in the hemoglobin solutions after chemical derivatization, Winterbourn equations were applied in the following form [Citation19]: [μM oxy] = 119* A577-39* A630-89*A560, [μM met] = 28*A577 + 307*A630-55*A560, [μM hemichrome] = -133*A577-114*A630 + 233*A560.
UV-vis spectra were recorded on Agilent 8453 (Agilent, Inc.) and Cary 50 (Varian, Inc) instruments. Dioxygen affinity and autooxidation measurements were in PBS pH 7.4 at room temperature (∼23 °C) and 37 °C, respectively.
For EPR spectra, a Bruker EMX Micro spectrometer with a liquid nitrogen cooling system was employed for EPR spectra. Instrument conditions were: microwave frequency 9.43 GHz, microwave power 15.89 mW, modulation frequency 100 kHz, modulation amplitude 5 G, sweep rate 22.6 G/s; time constant 81.92 ms, average of three sweeps for each spectrum, temperature 100 K. Sample volumes were typically 300-500 μL.
RESULTS AND DISCUSSION
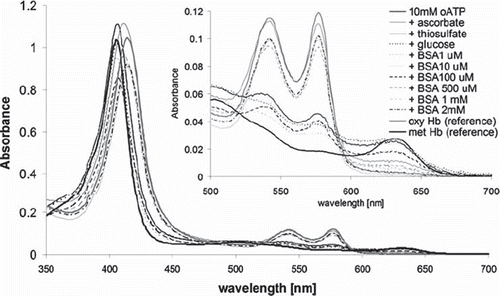
shows UV-vis spectra obtained immediately after derivatization of hemoglobin with oATP, with or without antioxidant agents. While oATP itself induces drastic autooxidation as witnessed in the increase of absorbance at 630 nm (diagnostic of ferric hemoglobin) and decrease in the 530-590 nm (region dominated by the oxy state of hemoglobin) [Citation18], all of the antioxidants employed here can alleviate this problem, to the extent wherein some of the samples the absorbance at 630 nm (hence, the autooxidation degree) is effectively zero.
Figure 1. UV-vis spectra of hemoglobin derivatized with oATP in the presence of various antioxidants; also shown for reference are spectra of underivatized oxy and met Hb. The spectra of derivatized samples are obtained by diluting equal amounts of each sample into 1 mL PBS. The concentrations of the oxy, met, and hemichrome forms (in this order, shown in parentheses, in micromolar) in each sample, calculated from Winterbourn equations as detailed in the Methods section, are as follows: 10 mM oATP (0.9, 7.0, 0.3), ascorbate (7.2, 2.9, 0.1), thiosulfate (6.8, 0.3, 0.0), glucose (0.0, 7.3, 0.9), 1 μM BSA (0.0, 6.6, 0.4), 10 μM BSA (0.4, 7.2, 0.4), 100 μM BSA (1.2, 4.9, 0.3), 500 μM BSA (5.8, 2.2, 0.0), 1 mM BSA (4.9, 1.2, 0.0), 2 mM BSA (6.7, 0.7, 0.0).
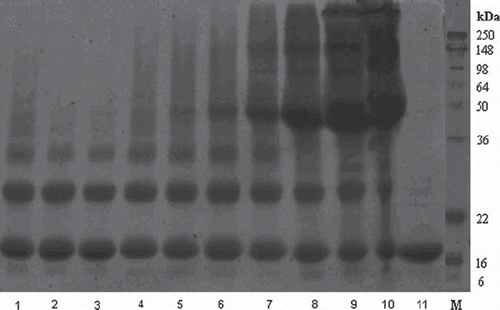
shows the SDS-PAGE analysis of the samples illustrated in . Treatment with 10 mM oATP (lane 1) induces several higher-molecular-weight bands compared to native Hb (lane 11), demonstrating that crosslinking has been induced by oATP. These diagnostic bands remain essentially unchanged in all the samples treated with antioxidants, suggesting that the decrease in autooxidation seen in is not accompanied by a decrease in crosslinking. Extra bands in lanes 5-10 are induced by BSA.
Figure 2. SDS-PAGE of oATP-derivatized hemoglobin in the presence of various antioxidants: 1-10oATP, 2- + ascorbate, 3- + thiosulfate, 4- + glucose, 5- + 1 μM BSA, 6- + 10 μM BSA, 7- + 100 μM BSA, 8- + 500 μM BSA, 9- + 1 mM BSA, 10- + 2 mM BSA, 11– pure Hb.
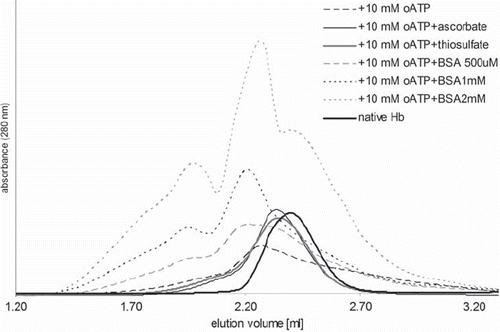
shows gel-filtration chromatograms illustrating that oATP induces an increase in molecular weight, and that this increase is partially conserved in the samples prepared with small-molecule antioxidants and is in fact amplified by BSA. Molecular weights calculated based on these chromatograms (cf. Materials and Methods) for the main peaks are 280 and 125 kDa, respectively, for the two major peaks of the BSA samples, and 75-125 for the other samples.
Based on the data from -, one can conclude that 1-2 mM BSA appears to be an optimal ingredient to completely avoid oxidation of hemoglobin during derivatization with oATP, while also maintaining a high molecular weight of the polymerization product. For these samples, autooxidation rates and oxygen binding curves were measured. lists these parameters, illustrating that the autooxidation rates of the copolymers are even lower than that of native hemoglobin, while oATP itself had an opposite effect. Furthermore, the dioxygen affinity and Hill coefficient are maintained at levels close to that of native hemoglobin. These features are particularly useful since chemical derivatization of hemoglobin, especially in the form of crosslinking, can lead to autooxidation, increased affinity, and decreased cooperativity [Citation4, Citation5, Citation9].
Table 1. Autooxidation rates (expressed in percentage increases relative to native hemoglobin), P50 values (also expressed as percentage increases), and Hill coefficients (n)for oATP-produced BSA-Hb copolymers.
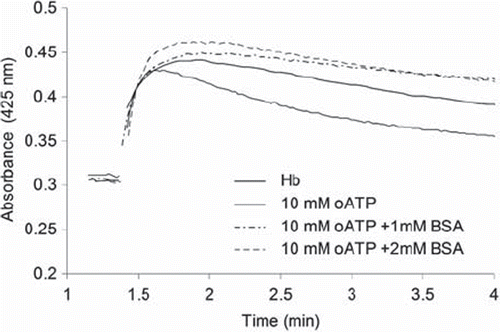
illustrates the time course at 425 nm during the reaction of hydrogen peroxide with oATP-polymerized Hb, with or without BSA. This wavelength is specific for the high-valent ferryl form of the heme (so-called Compound II), which is known to be involved in prooxidant reactivity of hemoglobin and myoglobin with medically relevant toxic effects [Citation20–22]; the fact that ferryl is formed even in crosslinked hemoglobins was verified by the typical UV-vis spectra, as well as by the fact that addition of sodium sulfide to ferryl solutions led to formation of a band at 620 nm, specific to the adduct formed between ferryl and sulfide (data not shown) [Citation18]. It can be seen from that the presence of albumin has led to a stabilization of the ferryl as witnessed by the fact that the absorbance remains even higher than that of native hemoglobin throughout the experiment. Perhaps relevant to this, addition of free BSA to native hemoglobin under the conditions shown in , in a 1:1 ratio, resulted in no change in the rate of ferryl decomposition and only a slight increase in the yield of formation (data not shown) – a behavior very similar to the derivatized hemoglobin prepared using oATP and BSA in a 1:1 ratio (sample labeled 10 mM oATP + 1mM BSA in ).
Figure 4. The reaction of derivatized hemoglobins with H2O2. Conditions: room temperature, 10µM Hb (native or subjected to derivatization with oATP and BSA at concentrations indicated), 100 µM H2O2, PBS 7.4.
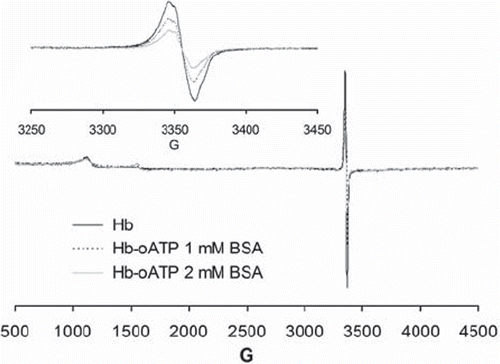
shows electron paramagnetic spectra (EPR) illustrating how copolymerization with BSA lowers the amount of free radicals generated upon reaction of hemoglobin with hydrogen peroxide: the intensity of the well-known hemoglobin radical signal at ∼3350 G is clearly lowered, in a dose-dependent manner, by copolymerization with BSA. This may be expected to be beneficial in a blood substitute, and is most likely due to radical quenching within the BSA aromatic and sulfur-containing aminoacids (including disulfide bridges). Other signals seen in the EPR spectra between 1000 and 1500 G are assignable to the ferric heme used as a starting material in the experiment, which is seen to be almost undetectable.
Figure 5. EPR spectra of 200 μM oATP-Hb and oATP-Hb-BSA copolymers treated with 500 μM H2O2 in PBS 7.4 and frozen at 20 s after mixing. Insert shows free radicals region of the samples (3300-3400 G, with intensities differing from sample to sample); the 1000-1500 G region shows small signals due to ferric iron, with similar intensities in all samples.
CONCLUSIONS
The data shown here indicate that copolymerization of hemoglobin with serum albumin alleviates prooxidant reactivity in hemoglobin during and after polymerization with periodate-oxidized ATP, to the extent where the copolymer even has a slightly lower autooxidation rate compared to native hemoglobin. A similar, although not as potent, effect is obtained when hemoglobin is derivatized with oATP in the presence of small-molecule antioxidants instead of albumin. A notable observation is that hemoglobin-albumin copolymers generated by reticulation with glutaraldehyde were previously shown to support respiration in a more efficient way than glutaraldehyde-polymerized hemoglobin [Citation23]. Our data now links these positive in vivo experiments with the fact that copolymerization with albumin leads to a decrease in prooxidant reactivity, and supports the concept that serum albumin should be considered as ingredient in hemoglobin-based blood artificial oxygen carriers. The antioxidant effect of albumin is likely due to its numerous sulfur-containing and aromatic aminoacids.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Alayash, A.I. (1999). Hemoglobin-based blood substitutes: Oxygen carriers, pressor agents, or oxidants? Nature Biotech, 17: 545–549.
- Alayash, A.I. (2000). Hemoglobin-based blood substitutes and the hazards of blood radicals. Free Radic. Res, 33: 341–348.
- Alayash, A.I. (2004). Oxygen therapeutics: can we tame haemoglobin? Nat Rev Drug Discov, 3: 152–9.
- Deac, F., Todea, A. and Silaghi-Dumitrescu, R. (2009). Metal Elements in Environment, Medicine and Biology Tome IX, Silaghi-Dumitrescu, R. Garban, G. (Cluj University Press, Cluj-Napoca, Romania), 165–173.
- Deac, F., Iacob, B., Fischer-Fodor, E., Damian, G. and Silaghi-Dumitrescu, R. (2010). Derivatization of hemoglobin with periodate-generated reticulation agents: evaluation of oxidative reactivity for potential blood substitutes. J Biochem (Tokyo), in press.
- Eike, J.H. and Palmer, A.F. (2004). Effect of Cl- and H+ on the oxygen binding properties of glutaraldehyde-polymerized bovine hemoglobin-based blood substitutes. Biotechnol Prog, 20: 1543–9.
- Eike, J.H. and Palmer, A.F. (2004). Effect of glutaraldehyde concentration on the physical properties of polymerized hemoglobin-based oxygen carriers. Biotechnol Prog, 20: 1225–32.
- Eike, J.H. and Palmer, A.F. (2004). Oxidized mono-, di-, tri-, and polysaccharides as potential hemoglobin cross-linking reagents for the synthesis of high oxygen affinity artificial blood substitutes. Biotechnol Prog, 20: 953–62.
- Eike, J.H. and Palmer, A.F. (2004). Effect of NaBH4 concentration and reaction time on physical properties of glutaraldehyde-polymerized hemoglobin. Biotechnol Prog, 20: 946–52.
- Dunne, J., Svistunenko, D.A., Alayash, A.I., Wilson, M.T. and Cooper, C.E. (1999). Reactions of cross-linked methaemoglobins with hydrogen peroxide. Adv Exp Med Biol, 471: 9–15.
- Dunne, J., Caron, A., Menu, P., Alayash, A.I., Buehler, P.W., Wilson, M.T., Silaghi-Dumitrescu, R., Faivre, B. and Cooper, C.E. (2006). Ascorbate removes key precursors to oxidative damage by cell-free haemoglobin in vitro and in vivo. Biochem J, 399: 513–24.
- Chang, T.M.S. (2009). Nanobiotechnology for hemoglobin based blood substitutes. Crit Care Clinics, 25: 373–382.
- Chang, T.M. (2004). Hemoglobin-based red blood cell substitutes. Artif Organs, 28: 789–94.
- Chang, T.M.S. (1997). Blood Substitutes: Principles, Methods, Products and Clinical Trials (Karger Landes, Basel).
- D’Agnillo, F. and Chang, T.M. (1998). Polyhemoglobin-superoxide dismutase-catalase as a blood substitute with antioxidant properties. Nat Biotechnol, 16: 667–71.
- D’Agnillo, F. Chang, T.M.S. (1998). Polyhemoglobin superoxide dismutase catalase as a blood substitute with antioxidant properties. Nat. Biotechnol, 16: 667–671.
- Chang, T.M., D’Agnillo, F., Yu, W.P. and Razack, S. (2000). Two future generations of blood substitutes based on polyhemoglobin-SOD-catalase and nanoencapsulation. Adv Drug Deliv Rev, 40: 213–8.
- Antonini, E. and Brunori, M. (1971). Hemoglobin and Myoglobin in their Reaction with Ligands (North-Holland, Amsterdam).
- Ibrahim, M.A., El-Gohary, M.I., Saleh, N.A and Elashry, M.Y. (2008). Spectroscopic study on oxidative reactions of normal and pathogenic hemoglobin molecules. Rom. J. Biophys, 18: 39–47.
- Reeder, B.J., Svistunenko, D.A., Cooper, C.E. and Wilson, M.T. (2004). The radical and redox chemistry of myoglobin and hemoglobin: from in vitro studies to human pathology. Antioxid. Redox Sign, 6: 954–966.
- Reeder, B.J., Grey, M., Silaghi-Dumitrescu, R.L., Svistunenko, D.A., Bulow, L., Cooper, C.E. and Wilson, M.T. (2008). Tyrosine residues as redox cofactors in human hemoglobin: implications for engineering nontoxic blood substitutes. J Biol Chem, 283: 30780–7.
- Silaghi-Dumitrescu, R., Reeder, B.J., Nicholls, P., Cooper, C.E. and Wilson, M.T. (2007). Ferryl haem protonation gates peroxidatic reactivity in globins. Biochem J, 403: 391–5.
- Fedorov, A.N., Iarochkin, V.S., Koziner, V.B., Hachaturian, A.A. and Rozenberg, G.I. (1978). Support of respiration and hemodynamics by complete blood replacement with artificial oxygen carriers: polyhemoglobinalbumin and polyhemoglobin. Dokl Akad Nauk SSSR, 243: 1324–6.