Abstract
Abstract: An ascorbate oxidase purified from the green fruit of zucchini squash (Cucurbita pepo medullosa) was immobilized photochemically on a polyethylene disc with 85% retention of initial activity of free enzyme. The optimum pH (5.5) was unchanged, while Km was decreased. The polyethylene discs were employed for determination of ascorbic acid in serum and foodstuffs. The working linear range was 2.8 μM to 16 μM. The mean value of ascorbic acid in serum as measured by the method was 0.16 mg/dl in males and 0.209 mg/dl in females. The immobilized enzyme was used 100 times over 4 months, when stored at 4°C.
INTRODUCTION
Ascorbic acid (Vitamin C), known for its reductive properties, has been used widely as an antioxidant in various food stuffs and drinks. It is important for therapeutic purposes and biological processes like production of collagen, and nor-epinephrine. Ascorbate is also an outstanding antioxidant in human blood plasma and causes stimulation of the pentose phosphate pathway and glutathione levels by dehydroascorbate (oxidized form of ascorbate).
The content of vitamin C in food stuffs reveals its nutritive value [Citation1]. Various methods are available for determination of ascorbic acid, such as the titrimetric method [Citation2], colorimetric method [Citation3], enzymic colorimetric method [Citation4], methanol method [Citation5], UV spectrophotometer [Citation6], HPLC [Citation7], sequential injection technique (SIA) [Citation8], and electrochemical methods based on ascorbate oxidase immobilized on CH-Sepharose via carbodiimide [Citation9] alkylamine glass beads [Citation10], ethylene vinyl acetate membrane [Citation11], sol gel silica matrix [Citation12], nylon net membrane [Citation13], glassy carbon electrode [Citation14], carbon paste modified electrode [Citation15], Pt micro electrode [Citation16], carbon nanotubes modified carbon fiber [Citation17], aminated glassy carbon electrode [Citation18], Cu(II) zeolite [Citation19], egg shell membrane [Citation20], and epoxy resin membrane [Citation21]. However, the methods used for immobilization of ascorbate oxidase such as adsorption after BSA-glutaraldehyde crosslinking, electrochemical entrapment, or covalent coupling were either-time consuming, involved multistep procedures and a number of chemicals, or had a risk of leaching of enzyme.
The photochemical method for covalent immobilization of proteins/enzymes onto inert support has overcome this problem [Citation22]. The present study describes the photochemical immobilization of ascorbate oxidase onto polyethylene discs and its application in determination of ascorbate in serum and various food stuffs.
MATERIALS AND METHODS
Chemicals and Reagents
Glutaraldehyde (25%) and bovine serum albumin (BSA) from Sigma, USA, and L-ascorbic acid from SRL, Mumbai, were used. 1-flouro, 2-nitro, 4-azido benzene (FNAB) FNAB prepared from 4-fluoro-3-nitroaniline (Sigma-Aldrich, USA) and polyethylene discs were received from Prof. P. Nahar, IGIB, N. Delhi. All other chemicals were of AR grade.
Extraction and Purification of Ascobate Oxidase
Fresh green fruits (15 cm in length) of zucchini (Cucurbita pepo medullosa) plants grown in a local field were collected during the month of July (30 + 5°C) in an ice bath. Fruits were weighed and washed in distilled water and stored at 4°C until use. About 3mm-thick skin of chilled zucchini fruits (3.5 Kg) was peeled off with the help of a sharp knife with a curved blade and homogenized in a chilled mortar with pestle. The homogenate was filtered through 3 layers of muslin cloth and its pH was adjusted to 7.0 by adding sodium tetraborate. The filterate was centrifuged at 15000 3 g for 30 min at 4oC and the supernatant was collected and treated as crude enzyme. It was tested for activity and protein. The crude enzyme was purified by 65%(NH4)2SO4 precipitation, gel filtration on Sephadex G-100 (1.4 × 23 cm) run in 0.05 M sodium phosphate buffer pH 7.0, and ion exchange chromatography on DEAE-Sephacel column (2.5 × 12.5 cm) using a linear gradient of 0.1 M to 0.6 KCl in 0.02 M potassium phosphate buffer (pH 6.7). The homogeneity of purified enzyme was checked in PAGE using AgNO3 as a protein stain.
Assay of Ascorbate Oxidase
The assay of ascorbate oxidase was carried out in the dark as described [Citation6] with slight modification. The reaction mixture contained 2.9 ml 0.1 M phosphate EDTA buffer pH 5.6 (concentration of EDTA 0.5 mM) and 0.1 ml 5 mM ascorbate. The reaction was started by adding 0.1 ml of crude enzyme (15000 × g supernatant) to the test. The increase in A265 was recorded against the blank immediately with respect to time in a UV visible spectrophotometer (Make: Shimadzu-1700). The blank contained all components of the reaction mixture except the enzyme, which was replaced by a reaction buffer. The unit activity of enzyme was calculated as follows:
[e = 13.386 (extinction coefficient of dehydroascorbate); total volume = 3.1; enzyme volume = 0.1ml] One unit of the enzyme is defined as the amount of enzyme required to oxidize one micromole of ascorbate per min per ml at 25°C. The protein content of various enzyme preparations was determined by the Lowry method using bovine serum albumin (BSA) as standard protein [Citation23].
Photo-immobilization of Ascorbate Oxidase onto Polyethylene Disc
The purified ascobate oxidase was immobilized covalently onto polyethylene discs using a photolinker, FNAB, as described [Citation22] with modification as follows.
Activation of Polyethylene Discs
The polyethylene discs (2 × 2 cm in size) were activated by 1-fluoro-2-nitro-4-azidobenzene (FNAB) by pouring a solution of FNAB in methanol (1.82 mg/50μl methanol) onto the discs and keeping them inside a dark fume cupboard. Methanol was allowed to evaporate slowly. The dry FNAB-coated discs were then irradiated by UV light at 265 nm for 20 min. The discs were washed with methanol and dried again. These activated discs were used as a support for immobilization of ascorbate oxidase.
Immobilization of Ascorbate Oxidase on Polyethylene Discs
The purified enzyme (2 ml) was spread evenly on the activated disc in a glass beaker with the help of an eppendroff pipette and kept at room temperature for 3 hr with occasional shaking after every 15 min. The unbound enzyme was removed and tested for activity and protein. The discs were washed 3–4 times with 0.1 M phosphate EDTA buffer, pH 5.6, and tested for activity. The discs containing immobilized enzyme were stored at 4°C until used.
Assay of Immobilized Ascorbate Oxidase
This assay was carried out in a similar manner to that described for the free enzyme except that the reaction buffer was increased by 0.1 ml and the free enzyme was replaced by a polythlylene disc containing immobilized enzyme. The reaction was started by dipping the discs into the reaction mixture. The increase in absorbance at 265 nm was recorded against the blank with respect to time and unit activity was calculated as described earlier.
Kinetic Properties of Ascorbate Oxidase
To standardize the optimal working conditions of polyethylene discs consisting of immobilized ascorbate oxidase, the following kinetic properties were studied.
Optimum pH. To determine the optimum pH of immobilized enzyme, the pH of reaction buffer was varied from pH 4.0 to 7.0 using 0.05 M sodium citrate phosphate buffer in the pH range 4.0 to 6.0 and 0.05 M sodium phosphate buffer in the pH range 6.5 to 7.0.
Effect of Substrate Concentration. To determine the effect of substrate concentration on the immobilized enzyme, different concentrations of ascorbic acid were added into the reaction mixture to give its final concentration in the range 2.8 μM to 20 μM.
Determination of Ascorbate in Serum and Foodstuffs with Discs/Immobilized Asorbate Oxidase
The discs/immobilized enzyme were employed for measurement of ascorbate in serum and various foodstuffs as follows.
Collection and Pretreatment of Serum. Fresh blood samples of apparently healthy males and females were collected from Pt. BDS PGIMS, Rohtak Hospital. The blood was centrifuged at 2000 × g for 10min. and the supernatant was collected, mixed with methanol (160μl), and centrifuged again at 16,000 g for 7 min in order to remove the protein debris completely, then supernatant was collected and stored at 4oC until use.
Collection and Pretreatment of Foodstuff. The fresh fruits, such as apples, grapes, kennu, oranges, and mausmi, and vegetables such as tomatoes, cabbages and spinach leaves were collected from the local market. These were washed with distilled water and dried with tissue paper. Each foodstuff was cut into small pieces with a sharp knife, homogenized in a chilled pestle and mortar, and filtered through a double layer of muslin cloth. The filtrate was diluted suitably with distilled water and concentration of ascorbic acid was determined as follows.
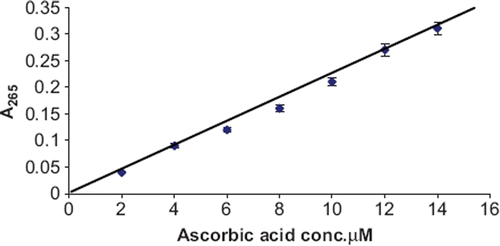
Determination of Ascorbate in Serum/Foodstuffs. The determination was carried out as described above for assay of immobilized enzyme under its optimal assay conditions, except that the ascorbic acid solution was replaced by serum or a suitably diluted foodstuff extract. The concentration of ascorbate in serum/ foodstuff was extrapolated from a standard curve between ascorbic acid concentration vs activity of photoimmobilized enzyme ().
Reusability and Storage of Discs/Immobilized Enzyme
To reuse the immobilized enzyme, the polyethylene discs containing immobilized enzyme were washed with distilled water 3–4 times followed by the reaction buffer, and then dried at room temperature. The discs were stored in the reaction buffer at 4°C when not in use.
RESULTS AND DISCUSSION
Purification of Ascorbate Oxidase from Cucurbita pepo medullosa
An ascorbate oxidase was purified from the green fruits of Cucurbita pepo medullosa using a combination of 65% (NH4)2SO4 precipitation, Sephadex G-100 gel filtration, and DEAE Sephacel ion exchange chromatography. The results () showed that the enzyme was purified by 43-fold with 18% yield. The purified enzyme exhibited a single band in PAGE (results not given).
Table 1. Purification of ascorbate oxidase from green fruits of zucchini squash (Cucurbita pepo Medullosa)
Photoimmobilization of Ascorbate Oxidase on Polyethylene Discs
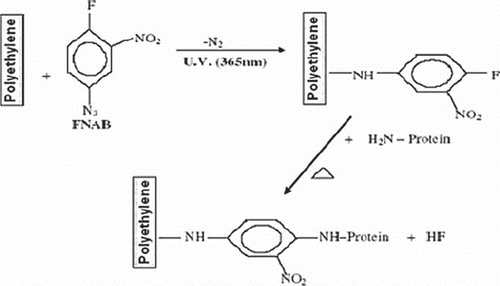
Purified ascorbate oxidase was immobilized covalently on polyethylene discs by 1-fluoro, 2-nitro, 4 azido benzene (FNAB) treatment under UV light with 85% retention of initial activity of free enzyme and a conjugation yield of 0.025 mg/4cm2. The chemical reactions involved in photoimmobilization are given in . The polyethylene disc is an inert support. During immobilization, the photolinker, FNAB, got attached to it (support) with the help of azide groups under UV light, and the free flouro groups of FNAB interact with free –NH2 groups on the surface of enzyme molecules [Citation16]. The present method of immobilization has advantages over earlier methods in that it is simple, rapid, and economic, as it requires only 4 hr, two chemicals (FNAB and methanol, which are cheap and readily available), and no special storage conditions for the immobilized enzyme. Further, the method involves no significant conformational change, which leads to good retention of enzyme activity upon immobilization.
Kinetic Properties of Immobilized Ascorbate Oxidase
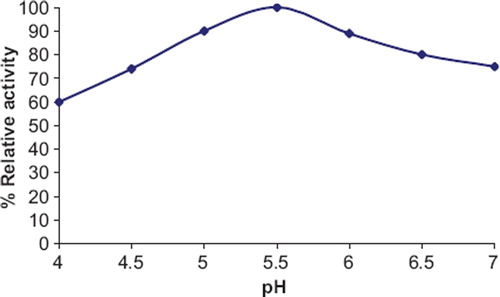
Effect of Optimum pH. The immobilized enzyme showed maximum activity at pH 5.5 (), which is similar to that of free enzyme (pH 5.5) [Citation6] and enzyme immobilized onto alkylamine glass beads [Citation10], but lower than that onto polyaniline film mounted on a Pt electrode [Citation16] and carbon paste electrode [Citation15].
Figure 3. Effects of pH on zucchini fruit ascorbate oxidase photo immobilized onto polyethylene disc.
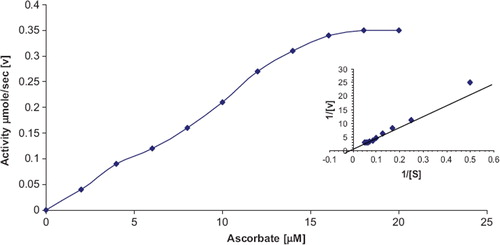
Effect of Substrate Concentration. The effect of ascorbic acid concentration on the initial activity of immobilized ascorbate oxidase was studied by varying the ascorbate concentration from 2.8μM to 20.0μM. A linear relationship was obtained up to 16μM, after which it was constant (). The Km value for ascorbate and Vmax as calculated from Lineweaver-Burk Plot ( inset) were 50.0 μM and 1.0 μmol/min, respectively. Km value of the immobilized enzyme was lower than that of the free enzyme (Km 126μM) [Citation6] and amperometric ascorbate oxidase biosensor [Citation24]. The change in kinetic properties of the enzyme after immobilization might be due to microenvironmental effects and bulk diffusion effects [Citation25].
Determination of Ascorbate in Biological Material with Polyethylene Discs/Immobilized Enzyme
An enzymic UV spectrophotometric method was developed for determination of ascorbate in serum and food stuffs employing polyethylene disc-bound ascorbate oxidase. In the method, A265 of dehydroascorbate generated from ascorbate by the immobilized enzyme was measured, which was directly proportional to ascorbate concentration. A linear relationship was found between A265 and ascorbic acid concentration ranging from 2.8 μM – 16.0μM (). The minimum detection limit of the method was 2.8μM. The method has the advantage that it is not only simple, sensitive, specific and rapid, but also provides the easy reuse of enzyme.
Ascorbate Level in Serum
The level of ascorbate concentration in serum as measured by the present method was in the range of 0.043 mg/dl to 0.21 mg/dl with a mean of 0.16 mg/dl for males and in the 0.043 mg/dl to 0.44 mg/dl with a mean of 0.21 mg/dl in females (). which are comparable to earlier reports [Citation13,Citation15, Citation26].
Table 2. Ascorbate level in serum by photo immobilized zucchini fruit ascorbate oxidase
Ascorbate Values in Foodstuffs
Ascorbic acid concentration in various fruits and vegetables as determined by the present method is given in . which was maximum in lemons and minimum in apples. These results are comparable to earlier reports [Citation27,Citation28].
Table 3. Ascorbate determination in some fruits and vegetables by photo immobilized zucchini fruit ascorbate oxidase
Reusability and Stability of Immobilized Enzyme
The immobilized enzyme was used for 100 times with only 30% loss of its initial activity over 4 months when stored at 4°C. The increased stability of enzyme immobilized on the polyethylene disc might be due to the microenvironment created by covalent bonding between the -NH2 groups of the surface of the enzyme and benzene ring of 2-nitro-4-azidobenzene attached chemically to the polyethylene disc (), which could stabilize the tertiary structure of the enzyme against chemical and physical denaturations [Citation29].
CONCLUSIONS
An ascorbate oxidase purified from zucchini fruits has been immobilized photochemically onto an inert support (polyethylene disc) using only two chemicals (FNAB and methanol). The discs measured L-ascorbic acid in serum and food stuffs. The discs/immobilized enzyme were highly stable and reusable. These characteristics make the photoimmobilized disc of ascorbate oxidase an attractive alternate to those who employ free/immobilized enzymes.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper
REFERENCES
- Puskas, F., Gergely, P., Perl, A. (2000). Stimulation of the pentose phosphate pathway and glutathione levels by dehydroascorbate. J FASEB, 14:1352–1361.
- Plummer, D.T. (1971). An Introduction to Practical Biochemistry, TMH, Tata McGraw-Hill Publishing Company, New Delhi, 229.
- Kum-Tatt, L., Leong, P.C. (1964). A new method for determination of ascorbic acid in urine. Clin Chem, 10: 575–580.
- Wimalasena, K., Dharmasena, S. (1993). Continuous spectrophotometric assay for ascorbate oxidase on a novel chromophoric substrate, 2-amino-ascorbic acid. Anal Biochem, 210:58–62.
- Badrakhan, C.D., Petrat, F., Holzhauser, M., Fuchs, A., Lomonosova, E.E., de Groot H., Kirsch, M. (2004). The methanol method for the quantification of ascorbic acid and dehydroascorbic acid in biological samples. J Biochem Biophys. Met, 58:207–218.
- Oberbacher, M.F., Vines, H.M. (1963). Assay procedure for ascorbate oxidase by UV spectrophotometer. Nature, 197: 1203–1204.
- Lykkesfeldt, J., Loft, S., Poulsen, H.E. (1995). Determination of ascorbic acid and dehydroascorbic acid in plasma by HPLC with colorimetric detection. Anal. Biochem, 229: 329–335.
- Polasek, M., Skala, P., Opletal, L., Jahodar, L. (2004). Rapid automated assay of anti-oxidation/radical-scavenging activity of natural substances by sequential injection technique (SIA) using spectrophotometric detection. Anal. Bioanal. Chem, 379:754–758.
- Stevanato, R., Avigliano, L., Finazzi-Agrò, A., Rigo, A. (1985). Determination of ascorbic acid with immobilized green zucchini ascorbate oxidase. Anal. Biochem, 149: 537–542.
- Marques, E.T., Lima-Filho, J.L. (1992). Ascorbic acid biosensor using ascorbate oxidase immobilized on alkylamine glass beads. Appl Biochem Biotechnol, 32: 73–78.
- Fernandes, J.C.B, Kubota, L.T., Neto, G.D.O. (1999). Potentiometric biosensor for l-ascorbic acid based on ascorbate oxidase of natural source immobilized on ethylene-vinylacetate membrane. Anal Chim Acta, 385: 3–12.
- Savini, I., Santucci, Di, R., Venere, A., Rosato, N., Strukul, G., Pinna, F., Avigliano, L. (1999). Catalytic and spectroscopic properties of cytochrome-c, horseradish peroxidase, and ascorbate oxidase embedded in a sol-gel silica matrix as a function of gelation time. Appl Biochem Biotechnol, 82:227–241.
- Tomita, I.N., Manzoli, A., Fertonani, F.L., Yamanaka, H. (2005). Amperometric biosensor for ascorbic acid. Eclet Quím, 30:37–42.
- Khorasai-Motlagh, M., Noroozifar, M. (2004). Electrocatalytical determination of ascorbic acid using glassy carbon modified with nickel (II) macro cycle containing dianionic tetraazaannulene ligand. Turk J Chem, 28:369–378.
- Fatibello-Filho, O., Vieira Iolanda da, C. (2000). L-ascorbic acid determination in pharmaceutical formulations using a biosensor based on carbon paste modified with crude extract of zucchini (Cucurbita pepo). J Braz Chem. Soc, 11: 412–418.
- Paixao, T.R.L.C., Lowinsohn, D., Bertotti, M. (2006). Use of an electrochemically etched platinum microelectrode for ascorbic acid mapping in oranges. J Agric Food Chem, 54: 3072–3077.
- Zhang, M., Liu, K., Xiang, L., Lin, Y., Su, L., Mao, L. (2007). Carbon nanotube-modified carbon fiber microelectrodes for in vivo voltammetric measurement of ascorbic acid in rat brain. Anal. Chem, 79:6559–6565.
- Wang, X., Watanabe, H., Uchiyam, S. (2008). Amperometric L-ascorbic acid biosensors equipped with enzyme micelle membrane. Talanta, 74:1681–1685.
- Tahereh, R., Taher, M.A. (2009). A new method for electrocatalytic oxidation of ascorbic acid at the Cu(II) zeolite-modified electrode. Talanta, 78:743–747.
- Chauhan, N., Dahiya, T., Priyanka, Pundir, C.S. (2010). Fabrication of an amperometric ascorbate biosensor using eggshell membrane bound Lagenaria siceraria fruit ascorbate oxidase. J Mol. Catal B. Enzymatic, 67(1–2): 66–71.
- Pundir, C.S., Chauhan, N., Jyoti (2010). Construction of an amperometric ascorbate biosensor using epoxy resin membrane bound Lagenaria siceraria fruit ascorbate oxidase. Artificial Cells, Blood Subst. Biotechnol, in press.
- Nahar, P., Naqvi, A. (2003). Method for preparing photo reactive polymers and immobilization of biomolecules onto these polymers. J Immunol. Met, 293:43–50.
- Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem, 193:265–275.
- Rekha, K., Gouda, M.D., Thakur, M.S., Karanth, N.G. (2000). Ascorbate oxidase based amperometric biosensor for organophosphorous pesticide monitoring. Biosens. Bioelectron, 15:499–502.
- Kennedy J P, Data on technique of enzyme immobilization and bioaffinity procedure. Handbook of Enzyme Biotechnology, Wiseman A. Second (Ellis Harwood Ltd., Chichester, UK) 1985, 394.
- Ihara, H., Shino, Y., Aoki, Y., Hashizume, N., Minegishi, N. (2000). A simple and rapid method for the routine assay of total ascorbic acid in serum and plasma using ascorbate oxidase and o-phenylenediamine. J Nutr Sci Vitaminol, 46: 321–324.
- Matsumoto, K., Yamada, K., Osajima, Y. (1981). Ascorbate electrode for determination of L-ascorbic acid in food. Anal. Chem, 53:1974–1979.
- Greenway, G.M., Ongomo, P. (2000). Determination of ascorbic acid in fruits and vegetables by flow injection with ascorbate oxidase. Anal. Biochem, 149:537–542.
- Ollis, D.F. (1972). Diffusion influences in denaturable insolubilized enzyme catalysts. Biotechnol. Bioeng, 14: 871–884.