Abstract
Abstract: Objectives: To investigate the cardioprotective effect of polymerized human placenta hemoglobin (PolyPHb) for acute myocardial ischemia rat heart. Methods: Myocardial infarcts (MI) model was set up in SD rats by permanent ligation of the left anterior descending coronary artery (LDA). The rats were divided randomly into 3 groups with each group of 20 rats: (A) the control group with the administration of Ringer's lactated solution at a dose of 2.5ml/kg); (B) PolyPHb group,PolyPHb solution at a dose of 0.16g Hb/kg and (C), PolyPHb+CAT+SOD group, PolyPHb solution at a dose of 0.16g Hb/kg and catalase (CAT) solution and superoxide dismutase (SOD) solution at a dose of 1681 U/kg and 528000 U/kg, respectively. Each rat received treatments via caudal vein, once a day for 7 days. Qualitative evaluations were made based on the reading of cardiac troponin T (cTnT), the myocardial infarction size (MIS) derived from the staining of myocardium tissue, and the pathological changes in infarct area. The ischemia changes of cardiomyocytes were determined by haematoxylin - basic fuchsin - picric acid (HBFP) staining and the apoptosis of cardiomyocytes were evaluated by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling assay (TUNEL). Results: Compared to the control group, PolyPHb greatly decreased the cTnT (p < 0.05), MIS (p < 0.05), and the size of myocardial ischemia (p < 0.05). PolyPHb + CAT + SOD decreased the △ST change (P < 0.05), cTnT (P < 0.01), MIS (p < 0.05), the pathological scores (p < 0.01), the size of myocardial ischemia (p < 0.01), and the apoptosis level (p < 0.01). Conclusion: Our study demonstrated that adding PolyPHb improves cardiac functional recovery and reduces myocardial infarction of rat heart.
INTRODUCTION
The main basic pathophysiology of myocardial infarction (MI) is the coronary artery occlusion, causing the reduction or interruption of blood supply to the myocardium, which in turn induces myocardial necrosis [Citation1].
Hemoglobin-based Oxygen Carriers (HBOCs) have been widely tested as red blood cell substitutes [Citation2–4]. As HBOCs have a higher oxygen-binding capacity, lower viscosities, and smaller diameters [Citation5–7] than red blood cells, they can pass through narrow openings in partially clogged blood vessels to transport oxygen to the oxygen-deficit tissues and thus reduce the ischemic injuries [Citation8–10]. Glutaraldehyde-polymerized human placenta hemoglobin (PolyPHb) is a particularly promising HBOC for treating ischemia as it has a high oxygen-carrying capacity and a low antigenicity. Furthemore, since red blood cells contain antioxidant enzymes such as catalase (catalase, CAT), superoxide dismutase (superoxide dismutase, SOD), and other enzymes for reducing free radical damages [Citation11], we added CAT and SOD as antioxidants to the oxygen-carrying PolyPHb. We tested the cardioprotective effect of PolyPHb + CAT + SOD on myocardial ischemia in the rats’ model.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats weighing 245–296 g (average wt, 262.0 ± 11.5g) were obtained from the Department of Laboratory Animal Science of Peking University Health Science Center, China. All animals were treated in accordance with the Guidelines on the “Use of Laboratory Animals” published by the National Institutes of Health. The protocol of the study was approved by local Animal Care and Use Committees.
PolyPHb and PolyPHb + CAT + SOD
PLP-polymerized placenta Hb in a buffered physiological solution of electrolytes was obtained from The Institute of Tianjin Union Biotechnology Development (Tianjin, China) [Citation5,Citation12]. CAT was obtained from Xinkai Long Biological Technology Co., Ltd. (Beijing, China); SOD was obtained from Sigma-Aldrich company (USA).
MI Protocol
MI was induced in adult male SD rats by ligating the LAD according to the technique of Pfeffer et al. [Citation13]. The animals were anesthetized intraperitoneally with 4.5% solution of sodium pentobarbital (1 ml/kg weight) and ventilated with room air by using a small animal ventilator (Zhejiang University apparatus).
The chests of rats were entered via a left lateral thoracotomy in the fourth intercostals space, the beating heart was visualized, and a ligature was placed around the LAD beneath the left auricle with a 4-0 silk suture. Successful performance of coronary occlusion was verified by blanching of the myocardium distal to the coronary ligation. All animals had free access to standard rat chow and water during the 7-day observation period.
The rats were divided randomly into 3 groups with each group of 20 rats: (A) the control group with the administration of Ringer's lactated solutionat a dose of 2.5ml/kg; (B) PolyPHb group, PolyPHb solution at a dose of 0.16g Hb/kg; and (C) PolyPHb + CAT + SOD group, PolyPHb solution at a dose of 0.16g Hb/kg and catalase (CAT) solution, and superoxide dismutase (SOD) solution at a dose of 1681 U/kg and 528000 U/kg, respectively. Each rat receives treatments via caudal vein, once a day for 7 days.
Electrocardiogram
Before the MI model, a standard lead II electrocardiogram (ECG) was performed to monitor the heart function of the rats in the supine position. Before the ECG measurements, each rat was anesthetized intraperitoneally with 4.5% solution of sodium pentobarbital (1 ml/kg weight).
Cardiac Enzyme Release Measurement
Blood samples were collected 24 hours after MI, and centrifuged at 4,000 rpm for 10 min. The plasma was stored at −20°C until further analysis. The release of cardia troponin T (cTn-T) was measured by Roche 2010 automatic electrochemiluminescence immunoassay analyzer.
Myocardial Infarction Size Determination
Myocardial infarction sizes in each group's hearts were estimated by nitroblue tetrazolium (NBT) staining. Briefly, for the postmortem, the heart was rapidly removed, and excess blood was rinsed off. Atrium and right ventricle were removed; left ventricle was cut into 0.1-cm-thick slices parallel to the atrioventricular groove. The slices were thawed and stained by incubation in 0.2% NBT solution in phosphate buffer (0.2M) at 37°C for 15min. Normal myocardium stained dark blue within 15 min, while the infarcts remained unstained or stained only faintly [Citation14]. The percentage of myocardial infarct size (MIS) (%) is calculated as the ratio of the weight of infracted myocardium over the weight of left ventricle [Citation15].
Myocardial Morphology and Pathology Analysis
The heart was used for paraffin embedment. Paraffin sections were stained by using hematoxylin and eosin (HE) to determine the morphology changes of cardiomyocytes.
Myocardial Ischemia and Apoptosis Determination
Paraffin sections were stained by using haematoxylin – basic fuchsin – picric acid (HBFP) to evaluate the ischemia changes of cardiomyocytes. The sections were photographed under light microscope (Olympus, Japan). The images were analyzed via Image-pro Plus 6.0 software.
For terminal dUTP nick end-labeling (TUNEL) assays, hearts were removed from the apparatus after MI, and the atrial tissue was dissected away. The ventricles were fixed in 10% buffered formalin and later embedded in paraffin according to standard procedures, and sections with 3μm thick were obtained to perform TUNEL assays using the ApopTag Plus Peroxidase In Situ Cell Death Detection Kit-POD (Roche Diagnostic, Germany) according to the manufacturer's instructions. TUNEL-positive myocytes were determined by randomly counting 10 fields of the left ventricular section and were expressed as a percentage of the total cardiomyocyte population.
Statistical Analysis
All values are presented as mean ± SEM. Statistical analysis was ANOVA for multiple comparisons. Values of P < 0.05 were considered statistically significant. Statistical analysis was performed using the SPSS statistical software package (version 17.0 for Windows).
RESULTS
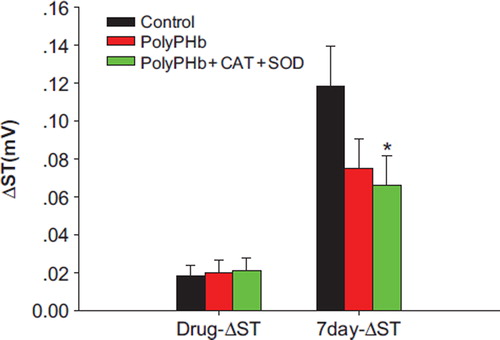
Effect of PolyPHb and PolyPHb + CAT + SOD Therapy on ST-wave Change in ECG
At the time of injection, the ST-wave changes among the 3 groups were the same (0.018 ± 0.006mV in control group, 0.020 ± 0.007mV in PolyPHb group, and 0.021 ± 0.007mV in PolyPHb + CAT + SOD group). However, at the end of the 7 days of MI, the ST-wave change of PolyPHb + CAT + SOD (0.067 ± 0.015 mV) group was significantly smaller than that in the control group (0.118 ± 0.021mV, P < 0.05); in the PolyPHb group, the recovery of ST-wave (0.075 ± 0.016mV) was better than that in the control group, but did not have statistical significance ().
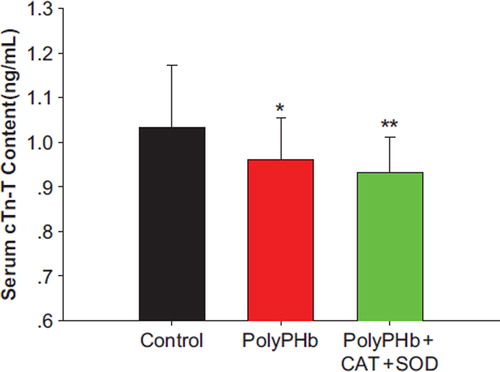
Cardiac Enzyme Release
In the PolyPHb and PolyPHb + CAT + SOD groups, the release of cTn-T into the circulation was greatly decreased (0.96 ± 0.09 ng/mL in the PolyPHb group and 0.93 ± 0.08 ng/mL in the PolyPHb + CAT + SOD group vs 1.03 ± 0.14 ng/mL in the control group, P < 0.05) ().
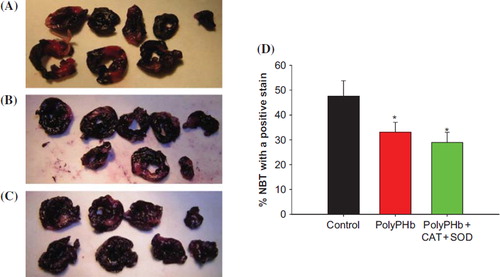
Myocardial Infarction Size (MIS)
In the PolyPHb and PolyPHb + CAT + SOD groups (33.04 ± 4.02% and 28.95 ± 4.07%, respectively, vs 47.54 ± 6.24% in the control group, p < 0.05), the infarct size was significantly decreased by PolyPHb and PolyPHb + CAT + SOD ().
Figure 3. Myocardial infarct size determined by NBT staining. Normal myocardium stained dark blue within 15 min, while the infarcts remained unstained or stained only faintly. NBT staining in control group (A); treated with PolyPHb group (B); treated with PolyPHb + CAT +SOD (C); and the percentage of myocardial infarct size (D). Values were expressed as mean ± SEM(n = 10). *P < 0.05 vs control group.
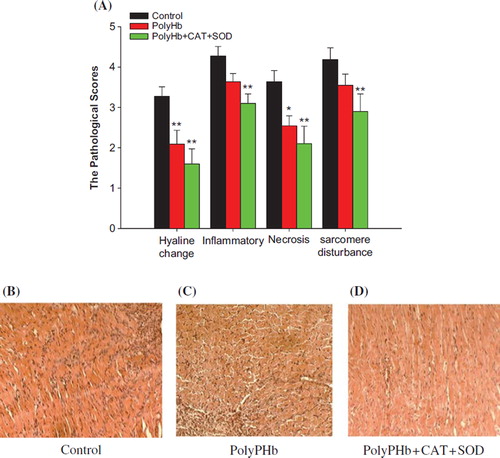
Myocardial Morphology and Pathology
The results of HE staining indicated that the hyaline change, inflammatory infiltration, necrosis, and sarcomere disturbance were significantly lessened in the PolyPHb + CAT + SOD ().
Figure 4. The statistical analysis of the HE-stained cardiac section (n = 10). The pathological picture in control group (B); treated with PolyPHb group (C); treated with PolyPHb + CAT + SOD (D); and the pathological scores (A). The pathological scores of the PolyPHb + CAT + SOD group were markedly reduced compared with the scores of the control group. Values were presented as mean ± SEM (n = 6). *P < 0.05, **P < 0.01 vs control group.
Myocardial Ischemia and Apoptosis
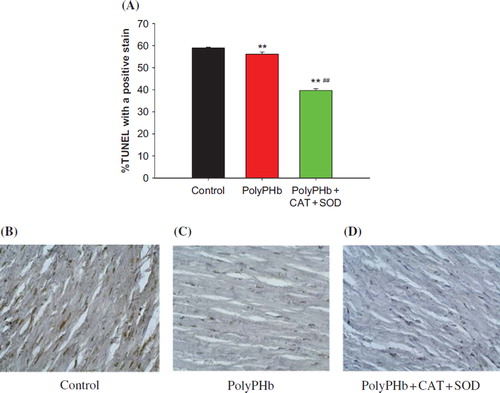
Ischemia changes of cardiomyocytes were determined by HBFP staining. shows the representative tissue sections of HBFP staining in each group. The size of myocardial ischemia in the control group was 38.41 ± 1.96%, which was greatly reduced to 32.74 ± 1.77% in PolyPHb group (P < 0.05 vs control group), and further decreased to 24.12 ± 1.52% in the PolyPHb + CAT + SOD group (P < 0.01 vs control group, P < 0.01 vs PolyPHb group). shows the representative tissue sections of TUNEL staining in each group. The density of the TUNEL-positive cells in the PolyPHb and PolyPHb + CAT + SOD groups were significantly smaller than that in the control group (56.12 ± 0.96 % in the PolyPHb group and 39.68 ± 0.83% in the PolyPHb + CAT + SOD group vs 58.88 ± 0.45% in the control group, P < 0.01).
Figure 5. Ischemia changes of cardiomyocytes determined by HBFP staining. Ischemic heart muscle, RBCs, collagen tissue stained with crimson red, while other tissues and structures stained with yellow. The HBFP staining picture in control group (B); in PolyPHb treated group (C); in PolyPHb + CAT + SOD treated group (D); and the pathological scores (A). Values were expressed as mean ± SEM (n = 10). *P < 0.05, **P < 0.01 vs control group. ##P < 0.01 vs PolyPHb group.
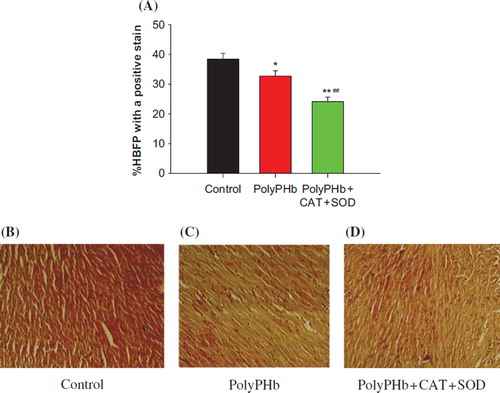
Figure 6. Apoptotic cells of cardiomyocytes determined by TUNEL staining. TUNEL detection in control group (B); in the group treated with PolyPHb (C); in the group treated with PolyPHb + CAT + SOD (D); and TUNEL positive cells ratio (A). The number of TUNEL positive vs control group when treatment with PolyPHb and PolyPHb + CAT + SOD at MI decreased. Values were expressed as mean ± SEM (n = 10). **P < 0.01 vs control group, ##P < 0.01 vs PolyPHb group.
DISCUSSION
This study demonstrates that HBOC can protect ischemic myocardium. First, HBOC can improve cardiac electrical activity. The ECG feature of cardiac injury is the ST-wave changes. After a 7-day MI, the ST-wave changes of PolyPHb + CAT + SOD and PolyPHb were significantly smaller than that of the control group. Second, HBOC reduces cardiac biochemical injuries. cTnT is a highly specific and sensitive cardiac enzyme. cTnT levels indicate the amount of myocardial injury. After 24h of MI, the release of cTnT into the circulation is greatly decreased in PolyPHb and PolyPHb + CAT + SOD groups. And most importantly, HBOC can significantly reduce the myocardial pathological damage. The percentage myocardial infarct size (MIS) is significantly decreased by PolyPHb and PolyPHb + CAT + SOD. The results of HE staining indicate that the hyaline change, inflammatory infiltration, necrosis, and sarcomere disturbance are also less in the PolyPHb and PolyPHb + CAT + SOD. In addition, the magnitude of myocardial ischemia and the apoptosis level are greatly reduced in PolyPHb group and in the PolyPHb + CAT + SOD group, which were shown by the reduced ischemia size during HBFP staining and the lowered number of the TUNEL-positive myocytes.
Compared with adult hemoglobin, the human placental blood hemoglobin has the following features: (1) Oxygen-carrying performance enhancement: P50 value of the placenta blood hemoglobin is 20 mmHg, while adult hemoglobin is 26mmHg; Hill coefficient of placental blood hemoglobin is 2.7, while adult hemoglobin is 2.8, oxygenation curve to the left, so that oxygen-carrying capacity has been increased. (2) Reduced antigenicity: Since the fetus developing is under the protection of the placental barrier, largely separated from the outside antigen, and the antigen protein is usually very weak, placental blood hemoglobin infusion could cause low receptor antibody titers, with rare occurrence of rejection and anti-host reaction [Citation16]. Therefore, we studied the PolyPHb expired animal blood hemoglobin and human hemoglobin in the blood oxygen-carrying capacity of a stronger and weaker antigen; tissue hypoxia is conducive to recovery. Compared with animal blood hemoglobin and human hemoglobin, PolyPHb has a higher oxygen carrying capacity and a weaker antigen and is beneficial to the recovery of hypoxia tissue.
Myocardial ischemia is myocardial hypoxia caused by hypoperfusion due to an imbalance of myocardial oxygen supply and demand. Inadequate myocardial perfusion may lead to the barrier of myocardial mechanical, biochemical and electrical activities, manifested as cardiac failure, angina, ventricular systolic dysfunction, and diminished cardiac pump function. PolyPHb diameter is 80–150 nm and its volume is only one percent of erythrocytes. The nano-sized PolyPHb is more likely to go through the occlusion blood vessels to provide oxygen to ischemic tissues.
Furthermore, the addition of antioxidant enzymes SOD and CAT greatly enhances the cardioprotective effects of HBOC. When hemoglobin is separated from red blood cells, the loss of protective effect of antioxidant defense system leads to the accumulation of free radicals, causing tissue oxidative damage. SOD can change superoxide into hydrogen peroxide, which is subsequently converted to water and oxygen by CAT, thus inhibiting free radical damages. Therefore, PolyPHb + CAT + SOD not only carries oxygen to tissues but also reduces reperfusion injuries in the tissues, thus it is a safer and more effective agent than PolyPHb in treating myocardial infarction.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Braunwald, E. (2001). Harrison's Principles of Internal Medicine, 15th ed. New York: McGraw-Hill.
- D'Agnillo, F., Chang, T.M. (1998). Polyhemoglobin-superoxide dismutase-catalase as a blood substitute with antioxidant properties. Nat Biotechnol. 16(7): 667–671.
- Burmeister, M.A., Rempf, C., Standl, T.G., . (2005). Effects of prophylactic or therapeutic application of bovine haemoglobin HBOC-200 on ischaemia-reperfusion injury following acute coronary ligature in rats. Br J Anaesth. 95(6): 737–745.
- Gannon, C.J., Napolitano, L.M. (2002). Severe anemia after gastrointestinal hemorrhage in a Jehovah's Witness: New treatment strategies. Crit Care Med. 30(8): 1893–1895.
- Li, T., Yu, R., Zhang, H.H., . (2006). A method for purification and viral inactivation of human placenta hemoglobin. Artif Cells Blood Substit Immobil Biotechnol. 34(2): 175–188.
- Standl, T., Freitag, M., Burmeister, M.A., Horn, E.P., Wilhelm, S., Am Esch, J.S. (2003). Hemoglobin-based oxygen carrier HBOC-201 provides higher and faster increase in oxygen tension in skeletal muscle of anemic dogs than do stored red blood cells. J Vasc Surg. 37(4): 859–865.
- McNeil, C.J., Smith, L.D., Jenkins, L.D., York, M.G., Josephs, M.J. (2001). Hypotensive resuscitation using a polymerized bovine hemoglobin-based oxygen-carrying solution (HBOC-201) leads to reversal of anaerobic metabolism. J Trauma. 50(6): 1063–1075.
- Deem, S., Kim, J.U., Manjula, B.N., . (2002). Effects of S-nitrosation and cross-linking of hemoglobin on hypoxic pulmonary vasoconstriction in isolated rat lungs. Circ Res. 91(7): 626–632.
- Levy, J.H., Goodnough, L.T., Greilich, P.E., . (2002). Polymerized bovine hemoglobin solution as a replacement for allogeneic red blood cell transfusion after cardiac surgery: Results of a randomized, double-blind trial. J Thorac Cardiovasc Surg. 124(1): 35–42.
- Moore, E.E., Johnson, J.L., Cheng, A.M., Masuno, T., Banerjee, A. (2005). Insights from studies of blood substitutes in trauma. Shock. 24(3): 197–205.
- Yang, C.M. (2001). Scientific Basis of Transfusion Medicine. Beijing: China Science and Technology Press.
- Chang, T.M.S. (2007). Artificial Cells: Biotechnology, Nanomedicine, Regenerative Medicine, Blood Substitutes, Bioencapsulation, Cell/Stem Cell Therapy. Hackensack, N.J: World Scientific.
- Pfeffer, M.A., Pfeffer, J.M., Fishbein, M.C., . (1979). Myocardial infarct size and ventricular function in rats. Circ Res. 44(4): 503–512.
- Nachlas, M.M., Shnitka, T.K. (1963). Macroscopic identification of early myocardial infarcts by alterations in dehydrogenase activity. Am J Pathol. 42: 379–405.
- Xu Shuyun, Bian Rulian, Chen Xiu (1994). Methodology in Pharmacological Experiment. Beijing: People's Medical Publishing House.
- Carter, A.M. (2009). Evolution of factors affecting placental oxygen transfer. Placenta. 30 Suppl A: S19–25.