Abstract
Abstract: In a previous dose escalation study our group found that combining 90μg/kg rFVIIa with HBOC-201 reduced blood loss and improved physiologic parameters compared to HBOC alone. In this follow-up study in a swine liver injury model, we found that while there were no adverse hematology effects and trends observed in the previous study were confirmed, statistical significance could not be reached. Additional pre-clinical studies are indicated to identify optimal components of a multifunctional blood substitute for clinical use in trauma.
INTRODUCTION
Trauma is the most frequent cause of death in Americans under the age of 34 years [Citation1]. Up to 50% of all early trauma deaths are due to uncontrolled hemorrhage [Citation2]. In Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF) the main cause of death in casualties with potentially survivable injuries has been hemorrhage [Citation3]. Of those who died, 50% suffered from non-compressible hemorrhage [Citation3]. Such wounds present a significant challenge to first responders and in-hospital care providers.
Methods that successfully halt or reverse coagulopathy associated with trauma early in the resuscitation process may reduce hemorrhage and the need for massive transfusions, potentially decreasing mortality.
The primary use of recombinant Factor VIIa (rFVIIa) (Novo Seven®, Novo Nordisk, Denmark) is to treat hemophilia, but it has also been shown to induce hemostasis in situations characterized by impaired thrombin generation such as platelet defects or dilutional coagulopathy developing as a result of trauma and extensive surgery [Citation4]. rFVIIa has been demonstrated to be effective in decreasing blood loss in models of hypothermic coagulopathic swine with grade V liver injury [Citation2,Citation5]. Several case reports have described the use of rFVIIa in surgical and trauma patients with acquired coagulopathy due to trauma [Citation6–8], and recently a phase 3 randomized clinical trial showed that rFVIIa reduced blood-product use but did not affect mortality [Citation9].
Hemoglobin based oxygen carriers (HBOCs) are solutions of acellular hemoglobin (Hb) that are universally compatible, have low infectious risk, may be room temperature stable with long shelf life, provide effective intravascular volume expansion with low volume, and have oxygen-carrying capability. In the trauma setting, the use of hemoglobin based oxygen carriers as resuscitation fluids may be appealing, particularly as bridging fluids during pre-hospital resuscitation and transport. The bovine polymerized hemoglobin based oxygen carrier (HBOC-201, OPK (formerly Biopure), Cambridge, MA) has been shown to be more effective in restoring mean arterial pressure to normal values while requiring significantly less fluid volume when compared to other low volume and standard resuscitation fluids [Citation10–14].
In a previous dose escalation study we found that in swine with severe hemorrhagic shock due to a grade III liver injury addition of 90μg/kg rFVIIa to HBOC-201 improved cardiac output and anaerobic metabolism. There were also trends to reduced hemorrhage volume and improved survival [Citation15,Citation16]. In this study, we investigated the combination of HBOC-201 and 90μg/kg rFVIIa in comparison to HBOC-201 alone for pre-hospital trauma resuscitation in a swine model of uncontrolled hemorrhage. We hypothesized that in this well-powered animal study pre-hospital resuscitation with HBOC-201 and rFVIIa combined would show statistically significant reduction in blood loss and improved physiologic and hematologic parameters and survival compared to HBOC-201 alone.
MATERIALS AND METHODS
The experiments reported herein were conducted according to the principles set forth in the “Guide for the Care and Use of Laboratory Animals,” Institute of Laboratory Animals Resources, National Research Council, National Academy Press, 1996. The study was approved by the Naval Medical Research Center/Walter Reed Army Institute of Research Institutional Animal Care and Use Committee, and all procedures were performed in an animal facility approved by the Association for Assessment and Accreditation for Laboratory Animal Care International.
Test Fluids
HBOC-201 is purified, filtered, stroma-free and heat-treated bovine hemoglobin that is polymerized by gluteraldehyde-crosslinking to form polymers ranging from 130-500 kDa MW. It is prepared in a buffer similar to lactated Ringer's solution (LR) containing 13 g Hb/dL.
Recombinant FVIIa is a recombinant form of the activated form of human factor VII (NovoNordisk, Copenhagen, Denmark). Each vial contains 2.4 mg of rFVIIa resuspended in 4.3 ml sterile water to give a final concentration of 0.56 mg/ml. Each HBOC-201 bag (250 ml) was admixed with 2.25mg of rFVIIa, resulting in fluid concentrations of 9 μg/ml. The appropriate number of bags was prepared under sterile conditions according to animal weight immediately before T0.
Study Design
There were two arms of this experiment, a “non-survival arm,” where animals were euthanized two hours after the start of the experiment, and a “survival arm,” where animals were recovered from anesthesia after two hours and monitored for 72 hours before they were euthanized. Animals were randomized to receive one of the following “pre-hospital” treatments: HBOC-201 (n = 30), HBOC-201 + 90 μg/kg rFVIIa (HBOC + rFVIIa; n = 24), or no treatment (NON; n = 4). This study was started as a non-survival study with a 2-hour observation period. Seven animals total were completed (5 with HBOC-201 and 2 with HBOC + rFVIIa). We then had the opportunity to give data derived from this study more power by making this a 72-hour survival study, while keeping the original 2-hour invasive monitoring phase. Subsequently, the number of additional animals in the survival arm was 25 for HBOC-201, 22 for HBOC + rFVIIa, and 4 for NON. The number of animals in the NON group was low because we observed a high mortality without resuscitation in this injury model, which did not justify additional sacrifice of animals in a study that was powered to detect differences between the two other groups. All data from the seven animals in the non-survival arm were included in the analysis of parameters up to two hours.
Animal Preparation
Fifty-eight farm-bred immature male and female Yorkshire swine (Animal Biotech Industries, Danboro, PA) weighing 25.7 ± 3 kg were fasted 12 hours prior to the experiment with free access to water. Anesthesia was induced with intramuscular ketamine (33 mg/kg) and atropine sulfate (0.05 mg/kg), followed by mask ventilation with isoflurane (3.0%) to facilitate endotracheal intubation. After intubation, isoflurane concentration was adjusted to a minimum alveolar concentration (MAC) reading of 1.8 at the anesthesia machine to maintain a surgical plane of anesthesia. After surgical instrumentation, isoflurane concentration was reduced to a MAC of 1.5. End-tidal CO2 (ETCO2) was monitored continuously (Ohmeda 7800 series ventilator; Datex, Madison, WI). Animals were ventilated if needed with a tidal volume of 5-10 mL/kg at a rate of 12-15 breaths per min adjusted to maintain ETCO2 between 35 and 40 mmHg. Intramuscular buprenorphine (0.01 mg/kg) was given 10 minutes before the incision.
Catheter Placement
Animals were placed in dorsal recumbency, and the right external jugular vein and carotid artery were isolated. A 9 French (Fr) introducer sheath was placed in the external jugular vein and a 7.5 Fr pulmonary artery catheter (PAC) (Edwards Life Sciences, Irvine, CA) was inserted for continuous hemodynamic and cardiac output (CO) monitoring, and sampling of mixed venous blood. An 18-gauge (Ga) angiocatheter (Cordis, Johnson and Johnson) was placed in the carotid artery for blood pressure monitoring and blood sampling.
Liver Injury
An upper midline laparotomy was done to gain access into the peritoneal cavity and expose the liver to define and visualize the four major lobes. A standardized liver injury [Citation10] was created by placing a ring clamp on the left lower lobe, ∼50% in width and ∼25% in length, adjusting for relative size of the liver of each pig. The clamp was closed and an 11 surgical blade was used to lacerate the lobe 50% in width until it reached the clamp (start of experiment: time 0 [T0]). The clamp was opened after one minute, and the remaining 50% of the lobe was resected. The excised liver section was weighed to determine section size relative to body weight to ensure standardization. The animals were allowed to bleed freely from the injury site and blood was removed via continuous suction from the abdomen away from the injury site.
Resuscitation
Animals were randomized to receive one of the following “pre-hospital” treatments: HBOC-201, HBOC + rFVIIa, or no treatment. Resuscitation fluids were given at a rate of 10ml/kg/min, beginning at T15. Animals received additional infusions at T30 and T45 for hypotension (MAP < 60 mm Hg) or tachycardia [HR > (baseline + 5%)] during the simulated pre-hospital phase.
Hospital Arrival
Hospital arrival and medical care capability were simulated at T60. Normal saline (NS) was infused for hypotension, tachycardia, or lactic acidosis; blood transfusion (whole blood [WB]) was given for anemia. Care was provided in this specific order to simulate a delay from emergency room (ER) arrival until surgical intervention and control of hemorrhage (∼15 minutes). At simulated operating room (OR) arrival at T75 the injury was repaired using Surgicel (Ethicon, Switzerland) followed by pressure with gauze for two minutes to stop any bleeding. If this did not control bleeding, interrupted suturing with 3-0 prolene was used. Upon definitive control of hemorrhage, the abdomen was closed with towel clamps until definitive wound closing.
Wound Closing
In the survival arm, the pulmonary artery catheter, arterial, ear vein, and urine catheters were removed and surgical sites were repaired. The external jugular vein introducer was secured in place with 0-silk interrupted sutures for post-operative blood sampling. The introducer was flushed with 10% citrated flush to prevent clotting. Bleeding from arterial catheter removal was controlled with 4-0 or 5-0 Prolene. Surgical sites were closed using an interrupted suture pattern in multiple layers.
Post-Surgical Provisions in the Survival Arm
Animals were recovered from anesthesia at the end of the two-hour acute study and were non-invasively assessed over the subsequent three-day period. At approximately seven hours following T0 and twice daily thereafter, animals’ vital signs were measured, venous blood gases analyzed, and overall health assessed. Animals were provided blood for anemia or saline for hypotension, tachycardia, or lactic acidosis via the external jugular vein introducer. Animals were given antibiotics (cefazoline IV, 13mg/kg, HIKMA-Farmaceútica; Terrugen, Portugal) and analgesia (buprenorphine IM,0.01 mg/kg, Rewckitt Benckiser Healthcare Ltd.; Hull, England), 2-3 times daily based on clinical signs associated with infection (hyperthermia, inappetance, recumbency) or pain (vocalization, reluctance to move, bruxism). Vomit incidence, activity level, and consumption were scored for each pig twice daily. At the end of the 72-hour observation period animals were euthanized and a full necropsy was performed by a veterinary pathologist, who was blinded to the resuscitation groups. Pathology data will be reported in a separate publication.
Data Collection
Standard invasive and non-invasive hemodynamic parameters were monitored for 120 minutes. Heart rate, respiratory rate, arterial blood pressure, pulse oximetry, transcuteneous oxygen pressure, CO, ETCO2 and pulmonary artery catheter parameters were documented every five minutes from T0-T60, and every 15 minutes thereafter. CO was measured continuously. Urine output (UO) and pulmonary artery wedge pressure (PAWP) were measured every 15 minutes. Arterial and mixed venous blood samples were collected from the femoral and pulmonary artery catheters, respectively. Blood loss was recorded from a scale each minute for the first 30 minutes and every five minutes thereafter via suction. Sponge weight was quantified in five-minute intervals. Blood gases analysis (arterial and mixed venous) was performed with an automatic analyzer (ABL 705, Radiometer, Copenhagen, Denmark). Standard hematologic and hemostasis profiles were obtained from citrated blood samples collected at T0, T15, T60 and T120. Complete blood counts (CBC) with differential were performed using a Pentra 60C+ cell counter (HORIBA-ABX, Irvine, CA). Coagulation was reported as prothrombin time (PT) and was measured using clot-based principles on a Stat Compact (Diagnostica Stago, Parsippany, NJ). Full clot formation dynamics were measured by Thromboelastography (TEG) using a TEG-5000 Haemostasis Analyzer (Haemoscope Corp., Niles, IL). In vitro bleeding time was measured by the closure time (PFA-CT) of an ADP/collagen coated capillary after aspiration of 800 μl citrated whole blood using a PFA-100 (Dade Behring, Deerfield, IL).
Statistical Analysis
Combined data from survival and non-survival arm was used up to two hours. After that only the survival arm data was used for statistical analysis. In a previously published dose escalation study [Citation15] the HBOC-201 group had an estimated blood loss of 27.5 ± 5.6 ml/kg, and the HBOC + rFVIIa group had an estimated blood loss of 19.4 ± 3.7 ml/kg. Based on these results, ≥24 animals per group have a 99% power to reach statistical significance on a two-sided 0.05. Fisher's exact test was used to calculate the power when comparing the survival rate between HOBC-201 and HBOC + rFVIIa groups. The log-rank test was used to compare the survival functions between the treatment groups; Fisher's Exact Test was used to compare the proportion of animals in each group surviving to 72 hours. Analysis of variance (ANOVA) was used to compare hematologic, physiologic and fluid measurements between treatment groups at discrete time points. T-tests were used to assess pairwise comparisons. P-values < 0.05 (two-sided) were considered statistically significant.
RESULTS
Baseline physiological parameters were similar between all groups (p > 0.05). All animals responded similarly to the liver injury.
Survival
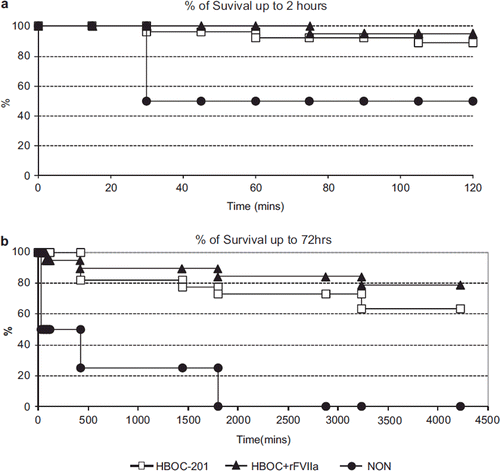
At hospital arrival at T60, 25/27 (96%) in the HBOC-201 group, 21/21 (100%) in the HBOC + rFVIIa group and 2/4 (50%) in the NON group survived (). At the end of the two-hour acute experiment phase, 24/27(89%) survived in the HBOC-201 group vs. 20/21(95%) in the HBOC + rFVIIa group (p = 0.42); 2/4 (50%) in the NON group survived (). The 72 hours survival rate was 64% (14/22) in the HBOC-201 group and 79% (15/19) in the HBOC + rFVIIa group (p = 0.30) (); no animals (0/4) in the NON group survived to 72 hours ().
Blood Loss Index
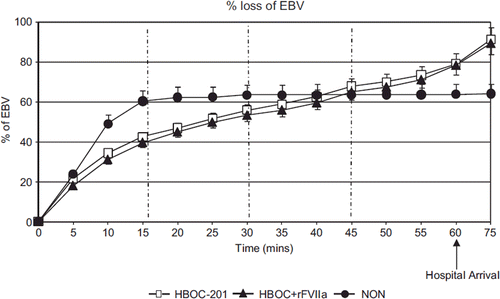
There was no statistical difference in percent loss of estimated blood volume (EBV) () between the HBOC-201 and the HBOC + rFVIIa group (p = 0.73). Blood loss in the NON group was higher than in the other two groups between T0 and T30 and lower between T60-T75 (p = 0.01, ). In the HBOC-201 group 6 out of 30 animals were ventilated, and in the HBOC + rFVIIa group 2 out of 24 animals were ventilated. There was no difference in bleeding between the non-ventilated and the ventilated subgroup within each group (p = 1.9).
Figure 2. The percent loss of estimated blood volume (EBV) () at T15 was 42%, 40%, and 60% for HBOC-201, HBOC + rFVIIa, and NON group, respectively (p = 0.002 HBOC + rFVIIa vs. NON). There was no statistical difference in percent loss of EBV () between the HBOC-201 and the HBOC + rFVIIa at hospital arrival T60 (79% and 78% p = 0.98).
Direct and Indirect Measures of Tissue Oxygenation
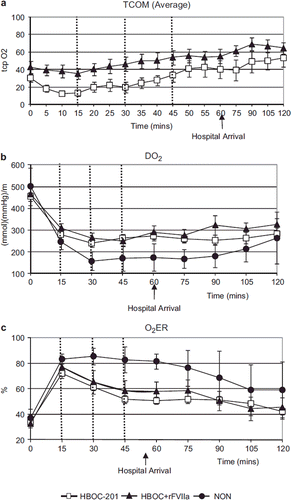
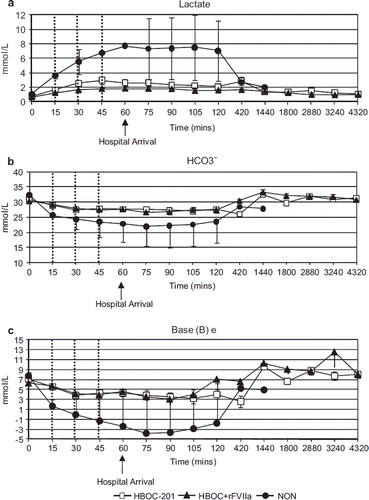
Transcutaneous oxygenation saturation (tcpO2) () was significantly higher over time in the HBOC + rFVIIa group compared to the HBOC-201 group (p = 0.01). However, the curves separated prior to initiation of treatments, confounding this result. O2 delivery (DO2), O2 consumption (VO2) and O2 extraction ratio (O2ER) (, ) were not significantly different between the HBOC-201 and the HBOC + rFVIIa groups, but were improved in both groups compared to the NON group (, p = 0.007 for O2ER). Indirect measures of tissue oxygenation [lactate clearance, HCO3−and Base Excess (BE)] were similar over time in both the HBOC-201 and the HBOC + rFVIIa groups, and were lower in the NON group (4a-c p < 0.0001 for lactate in T30). Addition of rFVIIa to HBOC-201 did not alter pH of the infused fluid (7.51 ± 0.01 for HBOC-201 and 7.48 ± 0.01 for HBOC + rFVIIa. Arterial blood pH remained unchanged during the experiment (7.42 ± 0.05 vs. 7.43 ± 0.06 for HBOC-201 and HBOC + rFVIIa groups). Glucose was also similar in both treated groups at all time points, but was significantly reduced compared to the NON group at T60 (3.1 ± 1.4 vs. 3.6 ± 1.4 vs. 8.5 ± 4.0 for HBOC-201, HBOC + rFVIIa, and NON, respectively).
Hemodynamics
By T60, MAP () had returned to baseline in the HBOC-201 (60 ± 4 mmHg) and the HBOC + rFVIIa (61 ± 5 mmHg) groups, whereas it did not return to baseline during the entire experiment in the NON (28 ± 6 mmHg) group (p = < 0.0001). In the HBOC-201 and the HBOC + rFVIIa groups, MPAP () reached baseline at T30 and remained above baseline until T60. In the NON group, MPAP remained below the baseline throughout the experiment (p = < 0.0001). HR increased in all groups in response to hemorrhage and began to normalize at T60 (). CI was similar in the HBOC-201 and the HBOC + rFVIIa groups and was restored to baseline at T120 minutes, whereas CI was lower in the NON group, never reaching the baseline ().
Figure 5. HR (Figure 5c) increased in all groups in response to hemorrhage and began to normalize at 60 minutes. MAP (Figure 5a), MPAP (Figure 5b), and CI (Figure 5d) was similar over time in both HBOC-201 and HBOC + rFVIIa groups and was significantly lower in NON animals compared to the other groups [MAP, (p < 0.0001)].
![Figure 5. HR (Figure 5c) increased in all groups in response to hemorrhage and began to normalize at 60 minutes. MAP (Figure 5a), MPAP (Figure 5b), and CI (Figure 5d) was similar over time in both HBOC-201 and HBOC + rFVIIa groups and was significantly lower in NON animals compared to the other groups [MAP, (p < 0.0001)].](/cms/asset/138779ee-2d3e-46f4-8b48-192e043116ca/ianb19_a_585615_f0005_b.gif)
Hematology
All three groups had similar hematology and coagulation parameters. As expected, PT () was shortened in the HBOC + rFVIIa group at T60 and T120 compared to the HBOC-201 group (13.0 ± 2.7 sec vs. 19.0 ± 4.4 sec respectively; p < 0.001, ANOVA). PT in the NON group remained unchanged from baseline. Due to the short half-life of rFVIIa, after 7 hours PT recovered to baseline in both treatment groups. PTT was unaffected by rFVIIa (). The reaction time (TEG-R) was shorter after injury and resuscitation (T60) compared to T0, corresponding to a mild hypercoagulation in both treatment groups (p < 0.01). TEG-R was lower at T60 and T420 in the HBOC + rFVIIa group (p < 0.01) and was higher in the NON group (). PFA and TEG-MA were unchanged from baseline in the NON group, but changed from baseline in both treatment groups (PFA in HBOC-201 was 62.9 ± 18.5 sec and 199 ± 87.2 sec in T0 and T60 respectively p < 0.01) (). This may be due to hemodilution caused by fluid resuscitation, which led to a decrease of Hct and platelets. The direct measurement of anti-thrombin III was impaired by HBOC-201 interference ; instead, the complex thrombin with anti-thrombin III (TAT) () was determined by ELISA as evidence of thrombin generation. TAT increased in the HBOC + rFVIIa group compared to the HBOC-201 group from T60 on (p < 0.01). NON animals were not tested as they died early. The other hematological parameters were unaffected by rFVIIa, but followed the expected pattern due to hemodilution by the resuscitation fluid (decreased values for Hct and platelets in pre-hospital phase). Hemodilution was not seen in NON animals (Hct and platelets remained stable). There were no platelet count differences between the treatment groups (); despite hemodilution, hemoglobin remained constant during most of the experimental time due to HBOC-201 infusion () with a decrease at T120 in both groups receiving HBOC-201; (p < 0.01); this was comparable to the level of Hb in the NON group.
Table 1. Parameters measured in swine treated with HBOC-201 or HBOC + rFVIIa during the experiment.
Fluid Requirements
In-hospital blood requirements were significantly less in both the HBOC-201 and the HBOC + rFVIIa animals compared to the NON group (p = 0.04). There were no differences in fluid and blood requirements between the HBOC-201 and HBOC + rFVIIa groups (p = 0.5).
DISCUSSION
This study is a more definitive investigation that complements a previous smaller dose escalation study wherein we compared different doses of rFVIIa with HBOC-201 and showed a trend towards decreased hemorrhage and more rapid reversal of anaerobic metabolism with HBOC + rFVIIa 90μg/kg (low dose) compared to HBOC-201 alone [Citation15, Citation16]. Based on data from the dose escalation study, this current study was well powered to compare differences in blood loss and survival between the HBOC-201 and HBOC + rFVIIa group.
We found expected improvement of in vitro measurements of coagulation (PT, TEG-R, TAT), but there were no significant group difference in hemorrhage volume, physiologic parameters, or survival. There was no statistical difference in survival with HBOC-201 vs. HBOC + rFVIIa. Overall our findings are consistent with the findings of our previous smaller study [Citation15, Citation16], as well as those of Schreiber et al. [Citation5]. Overall, the variations in hematology parameters were comparable to those observed in the dose escalation study [Citation16]. This study was conducted to support the previous finding that a low dose (90μg/kg) of rFVIIa was appropriate to use concurrently with an oxygen carrier in pre-hospital resuscitation for severe trauma. However, we were unable to establish superiority of adding rFVIIa to HBOC-201 infusion. Although there was no significant difference between groups, there was a trend towards better hemostasis (lower PT, TEG-R, and Lactate) and platelets were unaffected by rFVIIa. Of note, this study differed from the previous one by the species of animals used (Yorkshires vs. Yucatans) and the delay to hospital arrival (60 min vs. 4h). However, the results remained consistent. Adding a low dose of rFVIIa to a resuscitation fluid is not deleterious and may reduce co-morbidity.
Our findings are different from those of Schreiber [Citation5], Jeroukhimov [Citation17], Howes [Citation18], and Martinowtiz [Citation2] in the following criteria: (1) grade of liver injury; (2) time, dose, and number of administrations of rFVIIa; (3) splenectomy; (4) resuscitation fluid; (5) presence of hypothermia and coagulopathy. Schreiber et al. administered 150μg/kg of rFVIIa 30 seconds after grade V liver injury in swine. Although PT was decreased in the treatment group, there was no difference in blood loss compared to the control group. Our findings are consistent with theirs, although we administered a 90 μg/kg dose of rFVIIa mixed with HBOC-201 in repeated doses. Jeroukhimov et al. administered 180/720 μg/kg of rFVIIa as a bolus 30 seconds after grade V liver injury in swine. The group that received 180μg/kg did not show any improvement in blood loss and survival but the group that received 720 μg/kg showed statistical improvement in blood loss and survival [Citation17]. Howes et al. administered 120 μg/kg of rFVIIa as bolus 15 minutes after grade V liver injury, transverse femur fracture, and soft tissue injury of muscle in swine [Citation18]. The treatment group showed statistically significant reduction of blood loss compare to the control group. We administered a 90 μg/kg dose of rFVIIa mixed with HBOC-201 in three doses before simulated hospital arrival and eligibility to receive blood at 60 minutes. This may be one reason for not finding statistical significance in reduction of bleeding in our study.
Martinowtiz et al. administered 180 μg/kg of rFVIIa as a bolus 30 seconds after grade V liver injury in hypothermic and hemodiluted swine. The treatment group showed statistical reduction of blood loss compared to control. Unlike Martinowitz's study [Citation2], our animals were normothermic and non-coagulopathic at the time of the start of treatment. Also, our model is a grade III liver injury model, which is less severe than others, thereby allowing assessment of survival. This last factor may have constrained our power to demonstrate a treatment effect. Horton et al., stated that most clinical centers in the United States require some kind of blood product administration before giving rFVIIa, but there was a wide variation in the amount of required blood products [Citation19]. Once the decision to give rFVIIa was made, they found, institutions vary greatly on their dosing parameters for the drug. The 90 μg/kg dose in hemophiliacs is the only FDA-approved dose of the drug. An initial dose of 200μg/kg was used in the only randomized controlled trial of rFVIIa in the trauma population. However, only 3% of centers report using the 200 μg/kg dose, and only one-third use a dose greater than 90μg/kg. The 90 μg/kg dose of rFVIIa used in our study was derived from a previous dose escalation study performed in our laboratory [Citation15, Citation16]. Wide dose ranges for rFVIIa use have been described in the literature with inconclusive results (from as low as 5 mcg/kg to as high as 960 mcg/kg) [Citation2, Citation5, Citation8, Citation9, Citation15, Citation17, Citation18, Citation22, Citation23], and little data is available regarding pre-hospital use. This makes it difficult to predict the most effective dose of rFVIIa and further studies will be necessary to confirm potential benefit and dosage for pre-hospital use in humans.
Our study differed from current civilian clinical practice in that rFVIIa was evaluated for pre-hospital use, more than one additional dose was provided within the pre-hospital study period, and the drug was given over a period of 10 minutes. The value of repeated dosing has yet to be confirmed.
The majority of patients with combat-related injuries die from hemorrhage. The concept of damage control resuscitation and trauma-related coagulopathy has been reviewed previously [Citation20–22]. Hemostatic or damage control resuscitation can be considered the aggressive treatment of the early coagulopathy in trauma. This approach includes rapid control of bleeding, prevention and treatment of hypothermia and metabolic acidosis, minimizing dilutional coagulopathy by decreasing crystalloid and RBC transfusions, the empiric transfusion of plasma:RBC:platelets in a ratio of 1:1:1, and the early transfusion of fibrinogen and other pro-hemostatic agents in patients with life-threatening hemorrhage [Citation23].
Previous animal experiments have shown that HBOC-201, when given after controlled or uncontrolled hemorrhage, improved survival [Citation10, Citation12, Citation24]. There is also evidence that HBOC-201 improved cerebral perfusion pressure, brain oxygenation, reduced contusion size, and secondary hemorrhage in a polytrauma model combining blunt brain injury and hemorrhagic shock [Citation14, Citation25, Citation26]. Analysis of different end organs from animals of those studies showed minimal histopathologic changes indicative of clinically significant organ specific damage or increased oxidative stress at the cellular level due to HBOC-201[Citation11].
There are a few limitations of this study. First, study personnel were not blinded during the experiment. Second, the number of animals in the NON group was low because we observed high mortality with this injury model without resuscitation. Third, we did not include other controls such as resuscitation with lactated Ringer's solution or normal saline because the superiority of HBOC-201 over them has previously been established in the same model [Citation13, Citation14, Citation27] Fourth, severe coagulopathy of trauma was absent in our model. Fifth, while generally liked for its easy use and control, the inhalation anesthetic isoflurane has known vasodilatory effects. To reduce this potential confounder in our trauma study, we employed two strategies. First,we induced anesthesia with intramuscular injections of ketamine and buprenorphin, thus reducing possible pain perception. Second, after surgical instrumentation we titrated isoflurane to the lowest level possible without compromising the welfare of the animal. In our experience this was accomplished when the MAC reading at the anesthesia machine was 1.5. With these methods we accomplished comparable doses of the same potential confounder across all animals and groups.
CONCLUSIONS
In this swine model of severe hemorrhagic shock due to solid organ injury we found that repeat dose of 90μg/kg rFVIIa, when added to HBOC-201, did not show any statistical improvement in blood loss, physiologic parameters or, more importantly, survival. More research is needed to identify optimal components, their mixing ratio, as well as time and duration of application in the quest to create an effective multifunctional blood substitute for clinical use in trauma.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Office of Statistics and Programming, N. c. f. I. P. a. c., Center for Disease Control and Prevention. (2006). 10 Leading Causes of Death, United States. Centres for Disease Control and Prevention (CDC), Atlanta, GA, USA.
- Martinowitz, U., Holcomb, J. B., Pusateri, A. E., Stein, M., Onaca, N., Freidman, M., Macaitis, J. M., Castel, D., Hedner, U., Hess, J. R. (2001). Intravenous rFVIIa administered for hemorrhage control in hypothermic coagulopathic swine with grade V liver injuries. J Trauma 50: 721–729.
- Kelly, J. F., Ritenour, A. E., McLaughlin, D. F., Bagg, K. A., Apodaca, A. N., Mallak, C. T., Pearse, L., Lawnick, M. M., Champion, H. R., Wade, C. E., Holcomb, J. B. (2008). Injury severity and causes of death from Operation Iraqi Freedom and Operation Enduring Freedom: 2003-2004 versus 2006. J Trauma 64: S21-26; discussion S26–27.
- Hedner, U. (1998). Recombinant activated factor VII as a universal haemostatic agent. Blood Coagul Fibrinolysis 9 Suppl 1: S147–152.
- Schreiber, M. A., Holcomb, J. B., Hedner, U., Brundage, S. I., Macaitis, J. M., Aoki, N., Meng, Z. H., Tweardy, D. J., Hoots, K. (2003). The effect of recombinant factor VIIa on noncoagulopathic pigs with grade V liver injuries. J Am Coll Surg 196: 691–697.
- Dutton, R. P., Conti, B. M. (2009). The role of recombinant-activated factor VII in bleeding trauma patients. Curr Opin Anaesthesiol 22: 299–304.
- Martinowitz, U., Kenet, G., Segal, E., Luboshitz, J., Lubetsky, A., Ingerslev, J., Lynn, M. (2001). Recombinant activated factor VII for adjunctive hemorrhage control in trauma. J Trauma 51: 431–438; discussion 438–439.
- O'Neill, P. A., Bluth, M., Gloster, E. S., Wali, D., Priovolos, S., DiMaio, T. M., Essex, D. W., Catanese, C. A., Strauss, R. A. (2002). Successful use of recombinant activated factor VII for trauma-associated hemorrhage in a patient without preexisting coagulopathy. J Trauma 52: 400–405.
- Hauser, C. J., Boffard, K., Dutton, R. P., Bernard, G., Croce, M., Holcomb, J. B., Leppaniemi, A., Parr, M. J., Vincent, J. L., Tortella, B., Dimsits, J., Bouillon, B. (2010). Results of the control trial: Efficacy and safety of recombinant activated factor VII in the management of refractory traumatic hemorrhage. Western Trauma Association 40th Annual Meeting, Telluride, Colorado, 28 February – March 7, 2010. 117.
- Gurney, J., Philbin, N., Rice, J., Arnaud, F., Dong, F., Wulster-Radcliffe, M., Pearce, L. B., Kaplan, L., McCarron, R., Freilich, D. (2004). A hemoglobin based oxygen carrier, bovine polymerized hemoglobin (HBOC-201) versus Hetastarch (HEX) in an uncontrolled liver injury hemorrhagic shock swine model with delayed evacuation. J Trauma 57:726–738.
- Johnson, T., Arnaud, F., Dong, F., Philbin, N., Rice, J., Asher, L., Arrisueno, M., Warndorf, M., Gurney, J., McGwin, G., Kaplan, L., Flournoy, W. S., Apple, F. S., Pearce, L. B., Ahlers, S., McCarron, R., Freilich, D. (2006). Bovine polymerized hemoglobin (hemoglobin-based oxygen carrier-201) resuscitation in three swine models of hemorrhagic shock with militarily relevant delayed evacuation: Effects on histopathology and organ function. Crit Care Med 34: 1464–1474.
- Manning, J. E., Katz, L. M., Brownstein, M. R., Pearce, L. B., Gawryl, M. S., Baker, C. C. (2000). Bovine hemoglobin-based oxygen carrier (HBOC-201) for resuscitation of uncontrolled, exsanguinating liver injury in swine. Carolina Resuscitation Research Group. Shock 13: 152–159.
- Rice, J., Philbin, N., Handrigan, M., Hall, C., McGwin, G., Ahlers, S., Pearce, L. B., Arnaud, F., McCarron, R., Freilich, D. (2006). Vasoactivity of bovine polymerized hemoglobin (HBOC-201) in swine with traumatic hemorrhagic shock with and without brain injury. J Trauma 61: 1085–1099.
- Stern, S., Rice, J., Philbin, N., McGwin, G., Arnaud, F., Johnson, T., Flournoy, W. S., Ahlers, S., Pearce, L. B., McCarron, R., Freilich, D. (2009). Resuscitation with the hemoglobin-based oxygen carrier, HBOC-201, in a swine model of severe uncontrolled hemorrhage and traumatic brain injury. Shock 31: 64–79.
- Scultetus, A., Arnaud, F., Kaplan, L., Shander, A., Philbin, N., Rice, J., McCarron, R., Freilich, D. (2011). Hemoglobin-based oxygen carrier (HBOC-201) and escalating doses of Recombinant Factor VIIa (rFVIIa) as a novel pre-hospital resuscitation fluid in a swine model of severe uncontrolled Hemorrhage. Artif Cells Blood Substit Immobil Biotechnol. 39: 59–68. [Epub 2010 Jul 20].
- Arnaud, F., Hammett, M., Philbin, N., Scultetus, A., McCarron, R., Freilich, D. (2008). Hematologic effects of recombinant factor VIIa combined with hemoglobin-based oxygen carrier-201 for prehospital resuscitation of swine with severe uncontrolled hemorrhage due to liver injury. Blood Coagul Fibrinolysis 19: 669–677.
- Jeroukhimov, I., Jewelewicz, D., Zaias, J., Hensley, G., MacLeod, J., Cohn, S. M., Rashid, Q., Pernas, F., Ledford, M. R., Gomez-Fein, E., Lynn, M. (2002). Early injection of high-dose recombinant factor VIIa decreases blood loss and prolongs time from injury to death in experimental liver injury. J Trauma 53: 1053–1057.
- Howes, D. W., Stratford, A., Stirling, M., Ferri, C. C., Bardell, T. (2007). Administration of recombinant factor VIIa decreases blood loss after blunt trauma in noncoagulopathic pigs. J Trauma 62: 311–315; discussion 314–315.
- Horton, J. D., DeZee, K. J., Wagner, M. (2008). Use of rFVIIa in the trauma setting: Practice patterns in United States trauma centers. Am Surg 74: 413–417.
- Hess, J. R., Holcomb, J. B., Hoyt, D. B. (2006). Damage control resuscitation: The need for specific blood products to treat the coagulopathy of trauma. Transfusion 46: 685–686.
- Hess, J. R., Lawson, J. H. (2006). The coagulopathy of trauma versus disseminated intravascular coagulation. J Trauma 60: S12–19.
- Martinowitz, U., Michaelson, M. (2005). Guidelines for the use of recombinant activated factor VII (rFVIIa) in uncontrolled bleeding: A report by the Israeli Multidisciplinary rFVIIa Task Force. J Thromb Haemost 3: 640–648.
- Spinella, P. C., Perkins, J. G., McLaughlin, D. F., Niles, S. E., Grathwohl, K. W., Beekley, A. C., Salinas, J., Mehta, S., Wade, C. E., Holcomb, J. B. (2008). The effect of recombinant activated factor VII on mortality in combat-related casualties with severe trauma and massive transfusion. J Trauma 64: 286–293; discussion 293–284.
- Fitzpatrick, C. M., Biggs, K. L., Atkins, B. Z., Quance-Fitch, F. J., Dixon, P. S., Savage, S. A., Jenkins, D. H., Kerby, J. D. (2005). Prolonged low-volume resuscitation with HBOC-201 in a large-animal survival model of controlled hemorrhage. J Trauma 59: 273–281; discussion 281–273.
- Patel, M. B., Feinstein, A. J., Saenz, A. D., Majetschak, M., Proctor, K. G. (2006). Prehospital HBOC-201 after traumatic brain injury and hemorrhagic shock in swine. J Trauma 61: 46–56.
- Rosenthal, G., Morabito, D., Cohen, M., Roeytenberg, A., Derugin, N., Panter, S. S., Knudson, M. M., Manley, G. (2008). Use of hemoglobin-based oxygen-carrying solution-201 to improve resuscitation parameters and prevent secondary brain injury in a swine model of traumatic brain injury and hemorrhage: Laboratory investigation. J Neurosurg 108: 575–587.
- Arnaud, F., Handrigan, M., Hammett, M., Philbin, N., Rice, J., Dong, F., Pearce, L. B., McCarron, R., Freilich, D. (2006). Coagulation patterns following haemoglobin-based oxygen carrier resuscitation in severe uncontrolled haemorrhagic shock in swine. Transfus Med 16: 290–302.