Abstract
Abstract: We cultured isolated islets from human or porcine origin in the presence or absence of IL1 and TNFα and studied cytoprotective effects of two structurally different PBR ligands. Storage of pig or human islets in the presence of cytokines significantly lowered the fraction of vital beta-cells. Compared with cytokine incubations PK11195 alone or in combination with cytokines was effective to prevent cytokine induced cell death. The data indicate that cold storage in the presence of PK11195 may further protect beta-cells from cytokine induced cell death. This ligand may be helpful to preserve beta-cell survival before transplantation.
INTRODUCTION
One approach to treat type 1 diabetes is transplantation of islets of Langerhans [Citation1–3]. Tübingen University has established a clinical islet transplantation program on the basis of a GMP compliant manufacturing licence. In 2008, the first patient was successfully transplanted. According to the GMP manufacturing protocol, islets have to be stored and transplanted in Ringer-Lactat solution since cell culture media do not fulfill GMP requirements. With RL solution, islets easily disaggregate under cell culture conditions; therefore they have to be stored in the cold until all batch release criteria have been evaluated.
Cytokines (IL1ß or TNFα) are produced in isolated islets during cell culture. These cytokines are known to inhibit islet function due to direct interference with mitochondrial activity. Cytokines bind to membrane receptors and activate mitochondrial apoptotic pathways [Citation4,Citation5,Citation6]. The peripheral benzodiazepine receptor (PBR) is primarily located in the outer mitochondrial membrane of many cell types [Citation7,Citation8]. It can be detected in purified islets [Citation9,Citation10] and its expression is upregulated in islets after cytokine exposure [Citation11,Citation12]. As part of the mitochondrial permeability transition pore, the upregulation of PBR expression may be a mechanism of cytoprotection under conditions of sustained cell stress like islet isolation, hypoxia, or cytokine exposure [Citation7,Citation11,Citation12]. PK11195 or Ro5-4864 bind to mitochondrial PBR. These ligands inhibit mitochondrial activity but it is not known whether these ligands may also protect beta cells from cytokine-induced cell death.
In this study we examined the effect of cytokines and PBR ligands on islet viability during cold storage. The results obtained revealed a so-far unknown cytoprotective effect of PBR ligands, which may be of practical importance for manufacturing clinical islet transplants.
MATERIAL AND METHODS
Isolation of Porcine Islets
Since human islet preparations are scarce, we also performed islet isolations from porcine pancreata. This model has been established in our laboratory for many years [Citation13]. Porcine organs were taken from six-month-old, market-weight hybrid pigs (100 kg) immediately after exsanguination in the local slaughterhouse. After a warm ischaemic time of 15 min, the pancreata were purified from loosely adhering connective tissue and fat. The splenic lobe was dissected and injected via the pancreatic duct with 50 ml ice-cold University of Wisconsin solution (UW) to preserve the organ and to demonstrate the organ integrity [Citation14]. The digestion started three hours after organ procurement. Liberase (Roche, 1.5 mg/g tissue, 150 ml) was injected via the pancreatic duct and the organ was digested in a handmade Ricordi-chamber (1l HBSS, 37°C, manual shaking, mesh size 500μm, 8 stainless steel marbles) connected to a heating device. Islets were separated from exocrine tissue using a discontinuous Ficoll gradient in a COBE2991 cell separator. The isolation technique was performed using the recently described techniques [Citation15] modified by the closed system configuration [Citation13]. The closed system for islet isolation consists of the Ricordi-chamber, the cooling device, and the COBE2991 cell processor. In the closed system, the digest is flashed away from the Ricordi-chamber via the cooling device directly into the bag of the COBE2991 cell processor. The digest (600 ml) is immediately centrifuged and the bag is completely emptied, leaving the concentrated digest in the bag. This eluting procedure of the Ricordi-chamber is repeated until 3 L of new medium has passed the COBE. Islets are then purified using the modified UW/Optiprep discontinuous gradient [Citation15]. The bottom layer is added to the COBE bag and mixed for a period of 15 min with the digest (mix-function, COBE2991). After adding the middle and top layer, the tissue containing gradient is centrifuged. Finally fractions (20 ml) were taken from the gradient and screened for islets by dithizone staining. Islet yield is given as number of islet equivalent (IEQ) determined by areal density analysis [Citation16].
Isolation of Human Islets
Human pancreata were allocated to the Tübingen transplantation center. The preparation of islets followed the published standard technique [Citation17] with specific reference to GMP guidelines for aseptic processing of somatic cell therapeutics. The entire preparations were performed in a certified clean room laboratory class A/B. Medium for enzyme solution, digestion, and elution was HBSS supplemented with 25mM HEPES (Biochrom, Berlin, Germany). Islets were isolated by collagenase (NB1 GMPgrade, Serva, Heidelberg, Germany) and protease (NB1 GMPgrade, Serva, Heidelberg, Germany) digestion of the pancreas and separated from exocrine tissue using a continuous Biocoll density gradient (Biochrom, Berlin, Germany) in a COBE2991 cell separator. The islet yield and purity were determined by dithizone staining. In order to comply with GMP regulations, we used Ringer Lactat (RL) supplemented with 5% human albumin as culture medium.
Due to an insufficient number of isolated islets, the islet preparations used in this study were not used for transplantation and were therefore available for experimental purposes.
Cell Culture of Pig or Human Islets
Aliquots of purified pig islets (1500 IEQ/ml HAMS-F12, supplemented with 10% FCS) were cultured at 4°C in a refrigerator for 24 h. Aliqouts of purified human islets (1500 IEQ/ml) were cultured at 4°C for 24 h in Ringer lactate (RL) (Fresenius, Bad Homburg, Germany) supplemented with 5% human albumin (HA) (Biotest, Dreieich, Germany). These culture conditions resulted from restrictions of the supervising authority responsible for monitoring GMP compliance of cell therapeutics. Islets were cultured in the absence or presence of cytokines (IL1ß and TNFα at a concentration of 10 ng/ml each, Sigma-Aldrich, Steinheim, Germany) and of PBR-ligands (Ro5-4964, PK11195 at a concentration of 100 μM each, Sigma). After the indicated time intervals, islets were disintegrated into single-cell suspensions by incubating islet aliquots in 1 ml accutase solution (PAA Laboratories, Pasching, Austria) at 37°C for 15 min and dispersed by gently pipetting [Citation18].
Determination of Islet Cell Viability and Composition of Beta-cells from Pigs or Humans
Single-cell suspensions were incubated with 1 μM Newport Green PDX acetoxymethylether (NG; Molecular Probes) for 30 min at 37°C in PBS without Ca2+ and Mg2+. Newport Green allows for the definition of beta-cells according to zinc content [Citation18]. After washing, cells were stained with propidium iodide (PI; 10 μg/ml) (Sigma-Aldrich, Steinheim, Germany), a non-permanent dye that can penetrate only the membranes of dying/dead cells. Cell suspensions were analyzed using a FACSort cytometer (Becton Dickinson) with CellQuest and WinMDI Version 2.9 software.
Statistics
Values are given as mean ± SEM. For comparison of viable/dead cell ratios between the cytokine group (control) and the other groups of pig or human islets, repeated measures ANOVA with Dunnett multiple comparison test was used. A significant difference was assumed for values of p < 0.05.
RESULTS
Digestion and Islet Yield of Porcine Pancreata Using the Closed System
Using the newly developed closed system for islet isolation, a high islet yield with a high degree of purity was routinely obtained. shows purified porcine islets after dithizone staining. This specific preparation consists of large compact islets combined with low exocrine tissue contamination. Four pig pancreata were processed with an average weight of 74.5 ± 7.6 g. The mean digestion time was 31.5 ± 1.7 minutes, leaving a residue of undigested tissue of 19.5 ± 2.8 g in the Ricordi-chamber. The mean total islet yield after four purifications was 103897 ± 40147 IEQ, corresponding to 1631 ± 828 IEQ/g pancreas.
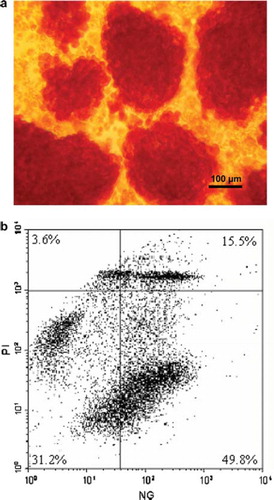
Figure 1. Purified pig islets after dithiozone staining and FACS analysis of freshly isolated purified porcine islets. a) Purified pig islets stained with dithiozone (original magnification 100x). b) Porcine islets (1500 IEQ/ml) were dispersed by incubating in accutase. Isolated cells were loaded with Newport-Green (NG) for beta-cell identification and with Propidium Iodide for discrimination of viable/dead cells. Dead beta-cells are found in the upper right quadrant of the dot-plot. Viable beta-cells are present in the lower right quadrant showing a bold population of living beta-cells. Percent values indicate the ratio of dots present in the indicated quadrants.
FACS Analysis of Porcine Islet Cells
shows an exemplified dot plot analysis by FACS of one preparation of freshly isolated and dissociated pig islets. This cell population consisted of 65.3% NG positive beta-cells of which 49.8% were NG positive and alive. Application of the FACS technique on four porcine islet preparations identified 67 ± 10% viable cells in all detected cell signals. 41.1 ± 12% of all cells were Newport Green (NG) positive and alive. For the determination of cytokine effects on the distribution of viable or dead beta-cells, the gate of detection was narrowed, thus allowing the analysis of a cell population consisting of 95% beta-cells. The effect of cytokines and PBR-ligands on the beta–cell survival was determined in this gate.
In the beta-cell specific gate the fraction of vital beta-cells determined 2h after cell preparation was 41.1 ± 12% in four preparations. Due to rapid islet dissociation and destruction of islets during cell culture at 37°C, porcine islets were stored in the cold. These storage conditions are similar to storage conditions of human islets described below. After 24h of cold-storage in the absence of cytokines, the fraction of vital beta-cells slightly increased from 41.1 ± 12% (freshly isolated islets) to 43.6 ± 5.14%.
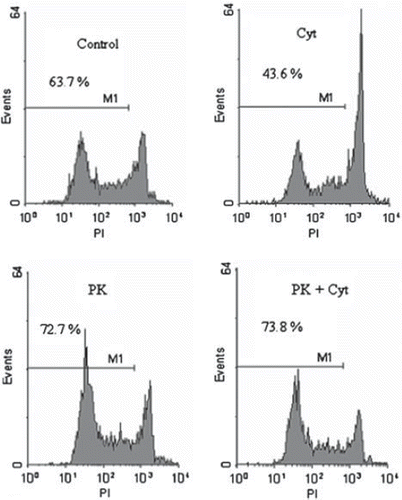
shows histogram plots from one exemplified experiment. Islets were stored in the cold in the presence or absence of cytokines or PK11195 as indicated. Addition of cytokines alone during cold storage significantly decreased the fraction of vital beta-cells from 63.7% to 43.6 %. Ro5-4864 (55.8%) was ineffective to prevent cytokine damage. Coincubation with PK11195 alone or in the presence of cytokines showed a clear protective effect (72.7% resp. 73.8% viable beta cells versus 43.6% in the cytokine group). The data show that cytokine incubation reduced the fraction of viable beta-cells. The PBR-ligand PK11195 was effective to completely prevent cytokine dependent cell death.
Figure 2. Histogram analysis of dissociated porcine beta-islet cells. Islets (1500 IEQ/ml) were cultured for 24h in HAMS-F12 at 4°C. Islets were co-incubated with cytokines (IL1ß and TNFα, 10 ng/ml each) or PK11195 at a concentration of 100μM. Islets were dissociated by accutase incubation and analyzed by FACS. M1 denote the proportion of viable beta-cells.
FACS Analysis of Human Islets
shows histogram plots of a FACS analysis of one exemplary human islet isolation. The human islet isolation consisted of 47.1% beta-cells. 67.4% of these beta-cells were PI negative and thus alive. Incubation of human islets with cytokines for 24 hours decreased the fraction of living beta-cells from 67.4 to 39.9%. PK11195 (69%) and Ro5-4864 (68.7%) alone had no effect on islet viability compared with cytokine-free incubation. PK11195 in combination with cytokines effectively protected beta cell survival (63.9%).
Figure 3. Histogram analysis of dissociated human beta-islet cells. Islets were cultured for 24h in RL/HA at 4°C. Islets were co-incubated with cytokines (IL1ß and TNFα, 10 ng/ml each) or PK11195 at a concentration of 100 μM. Islets were dissociated by accutase incubation and analyzed by FACS. M1 denote proportion of viable beta-cells.
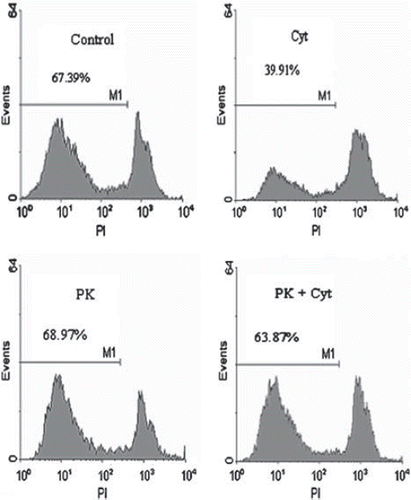
Thus, similar to porcine islets, cytokine exposure of human islet cells reduces the fraction of viable beta-cells. Co-culture of islets with cytokines and the PBR-ligand PK11195 effectively prevents cytokine-dependent cell death.
Effect of PBR Ligands on Pig and Human Islets
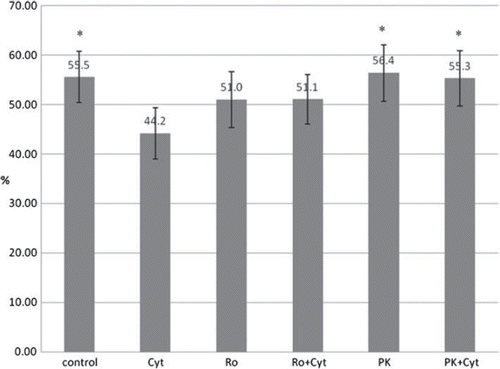
The effects of PBR ligands on either pig (n = 4) or human islets (n = 5) were very similar and could be summarized. Comparing all nine islet isolations (), the cytokine-treated group showed a significantly reduced viability compared to the control group (44.2 ± 5.1% vs 55.5 ± 5.2%, P < 0.01). Islets treated with Ro5-4864 alone or in combination with cytokines showed no significant difference compared with the cytokine-treated islets (51.0 ± 5.6% or 51.1 ± 5.0% vs 44.2 ± 5.1%). On the other hand, islet preparations treated with PK11195 alone or in combination with cytokines showed a significantly higher fraction of living beta-cells compared with the cytokine-treated group (56.4 ± 5.7% for PK11195 alone or 55.3 ± 5.6% for PK11195 combined with cytokines compared with 44.2 ± 5.1% observed in the cytokine group, P < 0.01). The data show the general effect of cytokine incubations reducing the fraction of viable beta cells. Only PK11195 but not Ro5-4864 is effective to completely prevent cytokine-dependent cell death.
Figure 4. Effect of PBR-ligands and cytokines on the viability of pig (n = 4) and human islets (n = 5) determined in FACS analysis. Values are given in percentage. Cyt: supplementation of cell culture medium with a combination of IL1ß and TNFα at a final concentration of 10 ng/ml each; Ro: supplementation of cell culture medium with Ro5-4864 at a final concentration of 100 μM; PK: supplementation of cell culture medium with PK11195 at a final concentration of 100μM. *:P < 0.01 for comparison of Cyt vs. control, Cyt vs PK and Cyt vs PK + Cyt after performing repeated measures ANOVA with Dunnett multiple comparision test.
DISCUSSION
In this study we examined beta-cell survival in the presence of IL1ß and TNFα, which activate mitochondria apoptotic pathways [Citation19–22]. These cytokines are produced by macrophages and directly by islets and may be important during organ procurement and islet preparation [Citation4–6]. With the specific PBR ligand PK11195 we were able to find similar cytoprotective effects in intact beta-cells from pigs and humans. On the other hand, with the structurally unrelated alternatively tested PBR ligand Ro5-4864 no cytoprotective effect was observed in either species. These data indicate that the specific cytoprotection of beta cells by the PBR-ligand PK11195 is species independent and may be of clinical relevance.
Our experimental protocol contained a period of cell storage in the cold for human or pig islets. We are obliged to store human islets in Ringer-Lactate solution until all batch release criteria are determined in order to fulfill GMP requirements for clinical islet preparation at the University of Tübingen. In RL solution human islets cannot be stored under cell culture condition because they rapidly disintegrate. However, storage in the cold maintains islet morphology and may protect cells from stress signals requiring metabolic activity. Low-temperature preconditioning is known to prevent cell damage in isolated islets of Langerhans [Citation23,Citation24]. In order to have comparable culture conditions for human and pig islets, we cultured porcine islets in the cold as well. Our attempts to use Ringer-Lactat for pig islet culture were, however, not successful, since porcine islets tend to dissociated under these conditions. We therefore incubated porcine islets in the cold with cell culture medium.
Although there are studies also adding IFNγ to the cocktail of cytokines our experimental protocol omitted INFγ. We excluded IFNγ since it has less or no influence on beta cell death, and is more involved in islet rejection after transplantation [Citation7].
Cytoprotections by the PBR-ligand PK11195, as shown in our study, are to some extent in contrast with recently observed pro-apoptotic effects in human islets [Citation25]. The reason for this discrepancy is not obvious, but differences in cell culture conditions (4°C in our study) and different concentrations of the PBR-ligands may be important. Similarly, heterogeneous results were reported regarding the effect of PBR ligands on cell survival ranging from protection to toxicity [Citation26]. Thus the mode of action of PBR ligands seems to be highly dependent on the chosen experimental conditions. Our data show that already after 24h in the cold, under conditions of reduced cellular metabolic activity, islet viability is significantly reduced by cytokines. Cytokine damage and cytoprotection by PK11195 may thus not require effective beta cell metabolism.
Human islet transplant protocols include at least a short period of islet culture before transplantation [Citation24]. According to our data, cytokine-induced beta-cell damage may occur within the timeframe usually needed for islet cell allocation to a recipient. This study therefore indicates that islet storage in the presence of the PBR ligand PK11195 may offer an advantage in islet mass preservation in this field.
CONCLUSION
In conclusion, the PBR-ligand PK11195 can prevent beta-cells from cytokine-induced cell death. This ligand may be useful during islet isolation and storage to prevent a decrease of the functional islet cell mass. Whether this form of pre-treatment affects islet survival after transplantation remains to be tested.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Ryan, E.A., Paty, B.W., Senior, P.A., Bigam, D., Alfadhli, E., Kneteman, N.M., . (2005). Five-year follow-up after clinical islet transplantation. Diabetes 54: 2060.
- Shapiro, A.M., Ricordi, C., Hering, B.J., Auchincloss, H., Lindblad, R., Robertson, R.P., . (2006). International trial of the Edmonton protocol for islet transplantation. N Engl J Med 355:1318.
- Ryan, E.A., Lakey, J.R., Paty, B.W., Imes, S., Korbutt, G.S., Kneteman, N.M., . (2002). Successful islet transplantation: Continued insulin reserve provides long-term glycemic control. Diabetes 51: 2148.
- Barshes, N.R., Wyllie, S., Goss, J.A. (2005). Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: Implications for intrahepatic grafts. J Leukoc Biol 77: 587.
- Eizirik, D.L., Mandrup-Poulsen, T. (2001). A choice of death – the signal transduction of immune mediated beta-cell apoptosis. Diabetologia 44: 2115.
- Abdelli, S., Ansite, J., Roduit, R., Borsello, T., Matsumoto, I., Sawada, T., . (2004). Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes 53: 2815.
- van der Windt, D.J., Bottino, R., Casu, A., Campanile, N., Cooper, D.K. (2007). Rapid loss of intraportally transplanted islets: An overview of pathophysiology and preventive strategies. Xenotransplantation. 14(4):288–97.
- Decaudin, D. (2004). Peripheral benzodiazepine receptor and its clinical targeting. Anticancer Drugs 15: 737.
- Bribes, E., Carriere, D., Goubet, C., Galiegue, S., Casellas, P., Simony-Lafontaine, J. (2004). Immunohistochemical assessment of the peripheral benzodiazepine receptor in human tissues. J Histochem Cytochem 52: 19.
- Giusti, L., Marchetti, P., Trincavelli, L., Lupi, R., Martini, C., Lucacchini, A., . (1997). Peripheral benzodiazepine receptors in isolated human pancreatic islets. J Cell Biochem 64: 273.
- Marchetti, P., Trincavelli, L., Giannarelli, R., Giusti, L., Coppelli, A., Martini, C., . (1996). Characterization of peripheral benzodiazepine receptors in purified large mammal pancreatic islets. Biochem Pharmacol 51: 1437.
- Trincavelli, M.L., Marselli, L., Falleni, A., Gremigni, V., Ragge, E., Dotta, F., . (2002). Upregulation of mitochondrial peripheral benzodiazepine receptor expression by cytokine-induced damage of human pancreatic islets. J Cell Biochem 84: 636.
- Klaffschenkel, R.A., Biesemeyer, A., Waidmann, M., Northoff, H., Steurer, W., Königsrainer, A., . (2007). A closed system for islet isolation and purification using the COBE2991 cell processor may reduce the need of clean room facilities. Cell Transplant 16: 587.
- Brandhorst, D., Iken, M., Tanioka, Y., Brendel, M.D., Bretzel, R.G., Brandhorst, H. (2005). Influence of collagenase loading on long-term preservation of pig pancreas by the two-layer method for subsequent islet isolation. Transplantation 79: 38.
- Krickhahn, M., Bühler, C., Meyer, T., Thiede, A., Ulrichs, K. (2002). The morphology of islets within the porcine donor pancreas determines the isolation result: Successful isolation of pancreatic islets can now be achieved from young market pigs. Cell Transplant 11: 827.
- Lembert, N., Wesche, J., Petersen, P., Doser, M., Becker, H.D., Ammon, H.P. (2003). Areal density measurement is a convenient method for the determination of porcine islet equivalents without counting and sizing individual islets. Cell Transplant 12: 33.
- Ricordi, C., Fraker, C., Szust, J., Al-Abdullah, I., Poggioli, R., Kirlew, T., . (2003). Improved human islet isolation outcome from marginal donors following addition of oxygenated perfluorocarbon to the cold-storage solution. Transplantation 75: 1524.
- Ichii, H., Inverardi, L., Pileggi, A., Molano, R.D., Cabrera, O., Caicedo, A., . (2005). A novel method for the assessment of cellular composition and beta-cell viability in human islet preparations. Am J Transplant 5: 1635.
- Mandrup-Poulsen, T., Bendtzen, K., Nielsen, J.H., Bendixen, G., Nerup, J. (1985). Cytokines cause functional and structural damage to isolated islets of Langerhans. Allergy 40: 424.
- Sandler, S., Eizirik, D.L., Sternesjö, J., Welsh, N. (1994). Cytokines and pancreatic B-cell function. Flatt, P., Lenzen, S. Frontiers of Insulin Secretion and Pancreatic B-Cell Research. London: Smith-Gordon, 553.
- Rey, C., Mauduit, C., Naureils, O., Benahmed, M., Louisot, P., Gasnier, F. (2000). Up-regulation of mitochondrial peripheral benzodiazepine receptor expression by tumor necrosis factor alpha in testicular leydig cells. Possible involvement in cell survival. Biochem Pharmacol 60: 1639.
- Sandler, S., Andersson, A.K., Barbu, A., Hellerström, C., Holstadt, M., Karlsson, E., . (2000). Novel experimental strategies to prevent the development of type 1 diabetes mellitus. Ups J Med Sci 105: 17.
- Cui, Y.F., Ma, M., Wang, G.Y., Han, D.E., Vollmar, B., Menger, M.D. (2005). Prevention of core cell damage in isolated islets of Langerhans by low temperature preconditioning. World J Gastroenterol. 11(4): 545–50.
- Noguchi, H., Naziruddin, B., Jackson, A., Shimoda, M., Ikemoto, T., Fujita, Y., . (2010). Low-temperature preservation of isolated islets is superior to conventional islet culture before islet transplantation. Transplantation. 89(1): 47–54.
- Marselli, L., Trincavelli, L., Santangelo, C., Lupi, R., Del Guerra, S., Boggi, U., . (2004). The role of peripheral benzodiazepine receptors on the function and survival of isolated human pancreatic islets. Eur J Endocrinol 151: 207.
- Hans, G., Wislet-Gendebien, S., Lallemend, F., Robe, P., Rogister, B., Belachew, S., Nguyen, L., Malgrange, B., Moonen, G., Rigo, J.M. (2005). Peripheral benzodiazepine receptor (PBR) ligand cytotoxicity unrelated to PBR expression. Biochem Pharmacol. 69(5): 819–30.