Abstract
Abstract: We prepared the sirolimus liposome by rapid expansion of supercritical solution (RESS) technology of supercritical fluid, and studied the effects of temperature, pressure, and equilibrium time on the average particle size and envelop rate of liposome. The conditions of the minimal average particle size of liposome were 328K of temperature (35MPa), 35MPa of pressure (333K), and 50 minutes of equilibrium (343K) time, respectively. The conditions of the maximal envelop rate of liposome were 333K of temperature (30MPa), 35MPa of pressure (343K), and 50 minutes of equilibrium (323K) time, respectively.
Keywords::
INTRODUCTION
Chloroform, ether, and methanol that are used in the process of traditional preparation of liposomes are harmful to humans and the environment. The liposomes prepared by the traditional method have wider scope of diameter, lower envelop rate, and are difficult to separate from the organic solvent. Traditional methods are not safe enough to some heat variable and bioactive substances [Citation1].
The supercritical fluid (SCF) is a sort of fluid that has critical temperature and pressure. The features of SCF are: (1) similar density to liquid, 100-fold more than gas, and there is obvious change of density as soon as there is a tiny change in temperature and pressure; (2) increased dielectric coefficient with increased pressure is favorable for the solublization of low-volatile substances; (3) similar viscosity to common gas, preferable flowability, penetrability, and transferability to common liquid.
Rapid Expansion of Supercritical Solution (RESS) is a method of preparation of microparticles. We dissolved the liposome materials in SCF, which has definite temperature and pressure, then sprayed the solution into lower pressure even vacuum through given nozzle in very short time (10−8-10−5S), the dissolving capacity of SCF takes place tremendous change because of the change of pressure, the degree of supersaturation of liposome solution is increased to 1000 folds, well-proportioned particle size liposome is formed, cross-linked in short time, and to obtain the better envelop rate of liposome. The characters of RESS process involve quick transitive perturbation and high supersaturation; the former results in uniform medium and narrow particle size distribution, the latter results in thinning microparticles. The SCF becomes a common gas after swelling; the pure gas can be used after purification and compression [Citation2].
MATERIALS
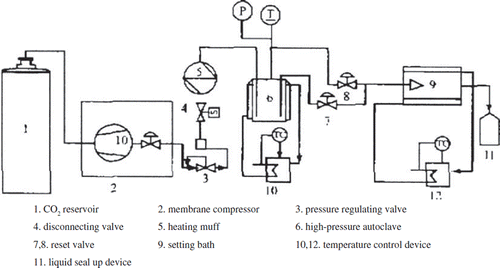
A high-pressure reaction kettle (200cm3, the precisions of thermograph and pressure gauge are 0.1°C and 0.01MPa, respectively, see ). CO2 (>99.9%) (Shanghai Lixin gas company). CO2 diaphragm type compressor (PPI corporation). Sirolimus, 20090402 (Lunan pharmaceutical corporation). Phospholipids, cholesterol (Sigma). Dialysis bag, mw 10000-14000 (Shanghai Yuanye biological company). PBS, (pH7.0, 200ml of 1.36g KH2PO4, 58.2ml 0.1M NaOH).
METHODS
We weighed 0.03g phospholipids and 0.015g cholesterol accurately, dissolved them with 10ml sirolimus solution (water solution with tween-80), vibrated by ultrasonic cleaner for 10 minutes, added ethanol into the above-mentioned solution, then put the solution into the reaction kettle, which had been blown with CO2. We raised the pressure to 25∼40 Mpa, and kept the pressure for 20∼60 minutes to form the white liposome solution.
Next, we put 1ml liposome solution into a dialysis bag, dialyzed in the beaker for 24 hours, then put 1ml dialysate in the refrigerator for determination [Citation3].
RESULTS
Effects of Temperature on Particle Size Distribution and Envelop Rate
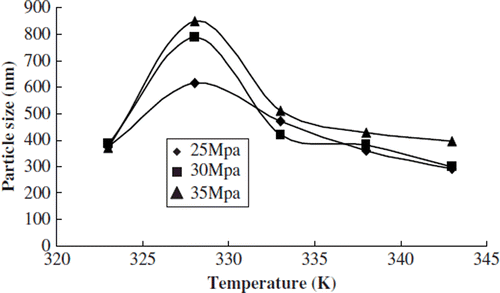
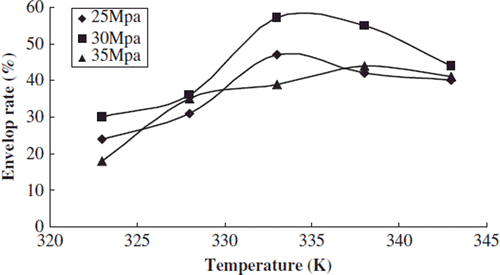
We studied the effects of temperature on the particle size distribution and envelop rate when the test pressure was 25, 30, and 35 Mpa, respectively. The content of phospholipids was 0.03g, and the concentration of sirolimus was 0.25 mol/L.
With different pressures conditions, the average particle size of liposome had a significant increasing trend when the temperature was raised from 323K to 328K. The average size had a significant decrease when the temperature was raised to 333K, and the average size had a slight decrease when the temperature was raised to 343K ().
With different pressures conditions the envelop rate of sirolimus liposome had a slight increasing trend when the temperature was raised from 323K to 328K. The envelop rate had a significant increase when the temperature was raised to 333K, and the envelop rate had a slight decrease when the temperature was raised to 343K ().
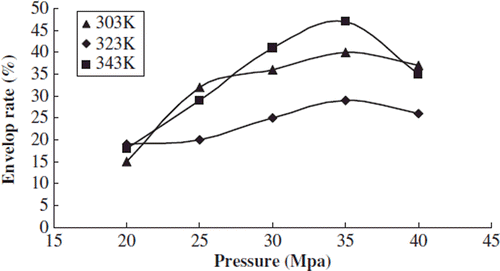
Effects of Pressure on Particle Size Distribution and Envelop Rate
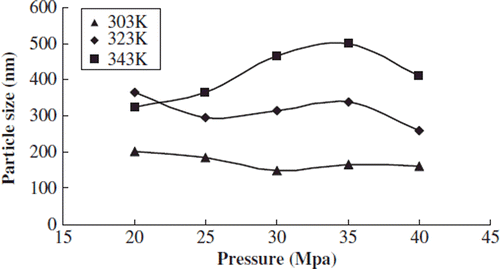
We studied the effects of pressure on the particle size distribution and envelop rate when the test temperature was 303, 323, and 343k, respectively, the content of phospholipids was 0.03g, and the concentration of sirolimus was 0.25 mol/L.
With different temperature conditions the average particle size of the liposome had a slight decreasing trend and slight fluctuation when the pressure was raised from 25MPa to 40MPa; the distribution of average size was 150nm∼500nm ().
With different temperature conditions the envelop rate of sirolimus liposome increased gradually when the pressure was raised from 25MPa to 35MPa; the envelop rate had a slight decreasing trend when the pressure was raised to 40Mpa ().
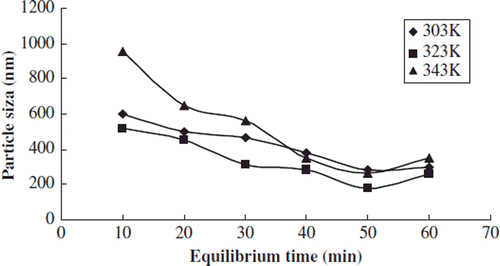
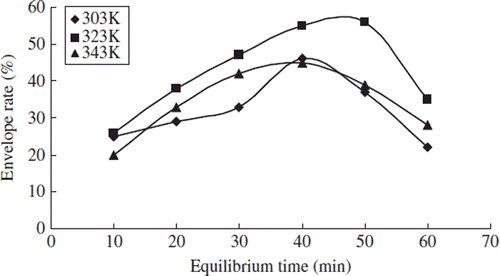
Effects of Equilibrium Time on Particle Size Distribution and Envelop Rate
We studied the effects of equilibrium time on the particle size distribution and envelop rate when the test temperature was 303, 323, and 343k, respectively, the test pressure was 35Mpa, the content of phospholipids was 0.03g, and the concentration of sirolimus was 0.25 mol/L.
The average particle size of liposome had a slight decreasing trend when the equilibrium time was in the range of 10min∼60min. The average particle size increased slowly after the critical point ().
The envelop rate of sirolimus liposome increased gradually when the equilibrium time was in the range of 10min∼40min; the envelop rate decreased gradually when the equilibrium time was in the range of 40min∼60min ().
DISCUSSION
The increasing temperature of the system aggravated the molecular random thermal motion and the flowability of double molecular layer of liposome in the microscopy view, therefore the average particle size of liposome increased. When the temperature reached the critical point, the average particle size got the maximum value, and the concentration of micelle in the system got the minimum value. Along with the increasing temperature, the flowability of the double molecular layer of liposome increased, resulting in the burst of the micelle to form a smaller-size micelle and smaller particle size of liposome [Citation4–6].
Along with the increasing temperature, the mode of lipid membrane was changed from gelatin to liquid crystal structure, the degree of irregularity and mobility of phospholipids chain and the flowability of lipid membrane increased, and the micelle fused to form a bigger liposome, resulting in the increasing envelop rate. When the temperature increased to the temperature of vitrificational transformation of phospholipids, the flowability of lipid membrane increased even more, resulting in the increasing release of enveloped drug and the decreasing envelop rate [Citation7].
On the condition of high pressures, the content of CO2 in the system increased. As a small molecule, CO2 could penetrate into the membrane of the swelled phospholipids double molecular layer, resulting in the burst micelle and the decreasing average particle size. The liposome micelle was a sort of solution system; the pressure had peak influence on the liquid system, so the continued increase of pressure resulted in the slight decreasing trend of average particle size [Citation8].
On the condition of original high pressures, more CO2 dissolved into the phospholipids membrane, was favorable to form a micelle, and resulted in the increasing envelop rate. Along with the constant increase of pressure, more and more CO2 penetrated into the membrane of the swelled phospholipids double molecular layer, resulting in the burst micelle, the overflow of drug, and the decreasing envelop rate.
The liposome micelle solution was a relatively stable system, whose overlong test time caused the decreasing liposome surface tension, resulting in the cross-linking, fusion of the molecules, and the increasing average particle size.
The molecules in the double layer of phospholipids had peak intermolecular acting force. Along with the overlong time, the surface tension decreased and the flowability of the double molecular layer increased, resulting in drug leakage and the decreasing envelop rate [Citation9].
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Kadimi, U.S., Balasubramanian, D.R., Ganni, U.R., . (2007). In vitro studies on liposomal amphotericin B obtained by supercritical carbon dioxide-mediated process [J]. Nanomedicine. 3(4): 273–280.
- Kompella, U.B., Koushik, K. (2001). Preparation of drug delivery systems using supercritical fluid technology [J]. Crit Rev Ther Drug Carrier Syst. 18(2): 173–199.
- Xie, F., Dong, Y., Liu, X., . (2006). Research progress in preparing microcomposites by supercritical fluid technology [J]. Modern Paint and Finishing, 9(12): 22–26.
- Manosroi, A., Chutoprapat, R., Abe, M., . (2008). Characteristics of niosomes prepared by supercritical carbon dioxide (sc CO2) fluid [J]. Int J Pharm. 352(1-2): 248–255.
- Pasquali, I., Bettini, R. (2008). Are pharmaceutics really going supercritical [J]? Int J Pharm. 364(2): 176–187.
- Tozuka, Y., Miyazaki, Y., Takeuchi, H. (2010). A combinational supercritical CO2 system for nanoparticle preparation of indomethacin [J]. Int J Pharm. 386(1-2): 243–248.
- Xie, F. (2009). Experimental Investigation on the Preparation of Hydrophobic Drugs Liposomes by Supercritical Technology. [D]. Dalian University of Technology.
- Padrela, L., Rodrigues, M.A., Velaga, S.P., . (2009). Formation of indomethacin-saccharin. Eur J Pharm Sci. 38(1): 9–17.
- Spence, A.J., Jimenez-Flores, R., Qian, M., . (2009). Phospholipid enrichment in sweet and whey cream buttermilk powders using supercritical fluid extraction [J]. J Dairy Sci. 92(6): 2373–2381.