Abstract
Abstract: To prepare an NCECS/nHA composite for tissue-engineered trachea and investigate its biomechanical and biocompatibile properties. Biomechanical tests were performed on dry and wet NCECS/nHA composite specimens prepared in vitro. The cell adhesion rate on each composite surface after 2, 6, and 12 hours of culture was calculated, and cell proliferation activity was measured using an MTT assay. NCECS/nHA composites exhibited satisfactory tensile strength and Young's modulus values. The adhesion rate of rabbit tracheal chondrocytes on NCECS/nHA surfaces reached 88.4% after 12 hours of culture. NCECS/nHA composites are promising scaffold materials for tissue-engineered trachea owing to satisfactory biocompatible and biomechanical properties.
INTRODUCTION
Due to the anatomical and physiological characteristics of the trachea, when the length of a circumferential sleeve resection exceeds the limitation of direct anastomosis (50 mm), only the implantation of a tracheal substitute can reconstruct the trachea to ensure an unobstructed airway [Citation1]. Tracheal substitutes include tracheal allografts, tracheal autografts, and artificial trachea. Currently used scaffold materials for tissue-engineered trachea primarily include synthetic polymers (such as polylactic acid, polyglycolide, and a polymer blend of the two) and natural polymers (such as collagen, alginate, and hyaluronic acid). The former show poor hydrophilicity and biocompatibility, whereas the latter show poor controllability during scaffold preparation, easily leading to changes in performance and functions. Therefore, a challenge remains to develop three-dimensional biomaterials with good biocompatibility for successful tissue-engineered trachea. The present study investigated an N-carboxyethylchitosan/nanohydroxyapatite (NCECS/nHA) composite in terms of biomechanical and biocompatibile properties as a potential biomaterial for tissue-engineered trachea.
MATERIALS AND METHODS
Materials and Instruments
New Zealand White rabbits (8 weeks old) were provided by the Laboratory Animal Center (Yangzhou University, China). DMEM/F-12 was purchased from Gibco (New York, NY, USA), fetal bovine serum (FBS) from Hangzhou Sijiqing (Hangzhou, Zhejiang Province, China), collagenase II from Gibco, and 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), trypsin, and ethylenediaminetetraacetic acid (EDTA) from Sangon Biotech Co. Ltd. (Shanghai, China). Chitosan was purchased from the China Huanyu Marine Biochemistry Company (China), and nHA was provided by Nanjing Emperor Nano Material Co. Ltd. (Nanjing, China). The WDW-5 Electron Universal Testing Machine was provided by the Hongzhan Group (Beijing, China). A FGC-0.2B pull and push dynamometer was purchased from Shimpo (Japan).
Isolation and Passage of Rabbit Tracheal Chondrocytes
Tracheal cartilage fragments (30 mm) from New Zealand White rabbits were cut into 1 mm3 blocks, washed three times with phosphate buffered saline (PBS) containing 100 U/mL penicillin, 100 mg/mL streptomycin and 25 μg/mL amphotericin B, and digested with 0.2% collagenase II in DMEM at 37°C for 5–8 hours. The digested cell suspension was screened with 150-mesh and 200-mesh stainless-steel filters in order to discard impurities. Subsequently, the resultant substance was centrifuged at 1000 r/min for 10 minutes, washed twice with PBS, and re-suspended in DMEM/F12 containing 272 μg/mL L-glutamine, 50 μg/mL ascorbic acid, and 10% FBS. After cell quantification, cell viability (>90%) was determined by trypan blue staining. The isolated tracheal chondrocytes were inoculated into a 75 mL culture flask at a density of 2.0 × 104/mL, and the culture medium was replaced every three days. Cells were passaged at 80–90% confluency.
Preparation of NCECS/nHA Composite
To prepare the NCECS/nHA composite, 2.5 g chitosan powder was added to 120 mL 5% acrylic acid solution. The mixture was stirred at 50°C until transparent and resultant NCECS was acquired 12 hours later. White flocculent precipitates were then prepared by adding a sufficient amount of acetone and washed at least three times with acetone. The NCECS powder was acquired after vacuum drying at 40°C. Next, 0.05 g nHA powder was scattered into 50 mL 2% NCECS solution during 24 hours of magnetic force stirring. The colloid solution was prepared into 5% NCECS/nHA solutions and then poured into a 24-well plate and freeze-dried to prepare porous scaffold thin films (). After sterilization by ethylene oxide, the resultant products were preserved at −20°C for later use.
Determination of Biomechanical Properties of NCECS/nHA Composites
The above thin films were trimmed into dumbbell shapes with a thickness of 100–150 μm and a width of 6.5 mm. Biomechanical tests were performed on dry and wet NCECS/nHA composite specimens. Two groups were included with six specimens per group: NCECS and NCECS/nHA. A WDW-5 Electron Universal Testing Machine was used for dry NCECS/nHA composite specimens. The dry specimens were fixed on the upward moving arm (1 mm/s) of the machine. The wet NCECS/nHA composite specimens were prepared by soaking NCECS/nHA composites in PBS for 24 hours, and the biomechanical properties were measured using a FGC-0.2B pull and push dynamometer. The tensile strength and Young's modulus of each specimen were determined.
Observation of Tracheal Chondrocyte Adhesion
To evaluate the adhesion of tracheal chondrocytes to the composite materials, NCECS/nHA, NCECS, and control (NCECS/nHA film-free culture plate) groups were evaluated (8 wells per group). After digestion, 1 × 105/L of passage 3 tracheal chondrocytes were inoculated into each culture plate (200 μL cell suspension/well) and cultured in a CO2 incubator. At 2, 6, and 12 hours after inoculation, the culture medium was removed, and non-adherent cells were removed using a PBS wash. Two wells of each group were digested with 0.7 mL digestion solution containing 0.25% trypsin and 0.02% ethylenediamine tetraacetic acid for 3–5 minutes. After resuspension, 0.3 mL culture medium containing serum was added to terminate the reaction. Adherent cells were quantified, and the cell adhesion rate was calculated.
Determination of Tracheal Chondrocyte Proliferation
Using the same experimental groups as above, 5.0 × 103 chondrocytes (0.1 mL) were seeded into a 96-well plate and a Petri dish of each group, and cultured under 5% CO2 at 37°C. At each time point (24 hours, 3, 5, 7, and 9 days of culture), one 96-well plate was removed and 20 μL (5 mg/mL) MTT solution was added. After an additional 4-hour incubation, the culture was terminated and the supernatant was discarded. Subsequently, DMSO (150 μl/well) was added to each well, and a 10-minute vibration was performed to thoroughly dissolve the crystals. The absorbance value at 490 nm was determined by an ELISA reader. At 9 days, cell proliferation in the Petri dish was evaluated using a scanning electron microscope.
RESULTS
Ultrastructure of NCECS/nHA Composites
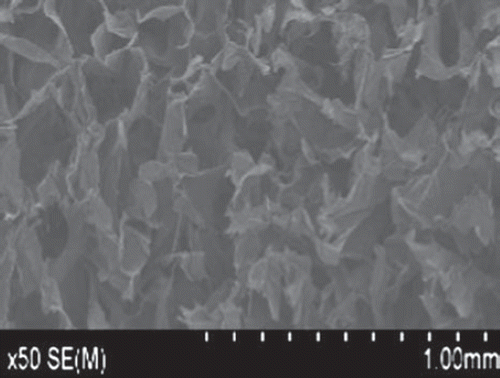
The NCECS/nHA composite scaffold exhibited high porosity, with the aperture size ranging between 300–600 μm ().
Biomechanical Properties of NCECS/nHA Composites
The NCECS/nHA composites, both under wet and dry conditions, exhibited better biomechanical properties in terms of tensile strength and elongation to break as compared to scaffolds of NCECS alone (P < 0.01) ().
Table 1. Biomechanical properties of NCECS/nHA composites
Adhesion of Tracheal Chondrocytes
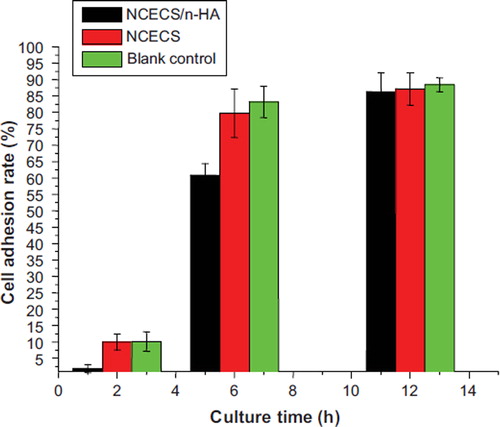
The cell adhesion rate of the tracheal chondrocytes decreased with the addition of nHA during early stages, but gradually increased with culture time. By 12 hours of culture, the cell adhesion rate was 86.33 ± 0.058% in the NCECS/nHA group, 87.2 ± 0.050% in the NCECS group, and 88.4 ± 0.021% in the control group. There was no significant difference between the groups (P > 0.05) ().
Proliferative Activity of Tracheal Chondrocytes on the Surface of NCECS/nHA Composites
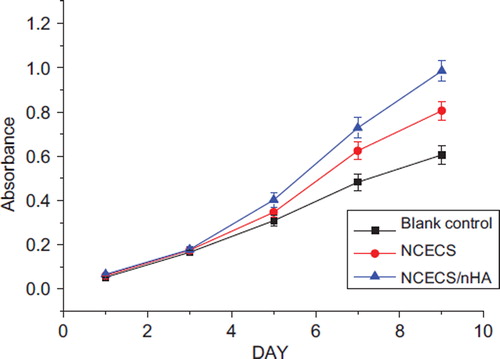
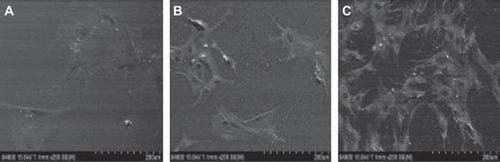
As shown in , the number of tracheal chondrocytes in each group increased with culture time. The growth curve of the tracheal chondrocytes exhibited an S-shape, corresponding to in-vitro growth characteristics of chondrocytes. The proliferative activity was significantly greater in the NCECS/nHA group than in the NCECS and control groups (P < 0.05). Scanning electron microscopy revealed that tracheal chondrocytes grew well on the surface of NCECS/nHA composites. Cells in the NCECS/nHA group exhibited higher cell density and better morphologies than in the NCECS/nHA and control groups ().
DISCUSSION
Hydroxyapatite (HA), the main inorganic constituent of human bone, has unique biological properties at nanometer-sized levels. Not only does nanohydroxyapatite (nHA) exhibit characteristics of nanometer biomaterials, but it also has excellent biocompatibility, thus contributing to its wide range of applications within the field of biomedicine. After implantation into the human body, nHA can directly integrate with bone tissue via chemical bonds due to its osteoconductive properties, providing an excellent physiological scaffold for new bone deposition and regeneration [Citation2–4]. However, its clinical application is restrained due to brittleness, difficult fabrication, and an extremely low degradation velocity, although these properties can be improved by combination with other materials [Citation5]. Chitosan is synthesized by removing acetyl groups from chitin. The degradation products of chitosan include aminoglucose, which is non-toxic, harmless, and a non-irritant [Citation6]. Chitosan exhibits excellent biocompatibility and can also promote wound and bone injury healing by stimulating the host organism to produce regenerative cells [Citation7–10]. Chitosan cannot be used for repair in complex regions, however, because it degrades rapidly in physiological environments and shows low mechanical strength.
Recently, chemical modifications of chitosan have become a rapidly growing area of research. Chitosan can acquire more functional activities by introducing other groups at the repetitive units [Citation11]. Evidence exists that, with increasing carboxyethyl substitution, chitosan shows gradually enhanced adhesion to metal ions and increasing surface hydrophilicity [Citation12]. N-Carboxyethylchitosan (NCECS) is an amphiphilic chitosan derivative. Our previous findings confirmed that the maximum temperature for in vitro degradation of chitosan was 254°C, whereas NCECS was 523°C, the latter providing a much slower degradation rate of chitosan [Citation13]. Additionally, NCECS has been reported to exhibit better biocompatibility than NCECS [Citation14].
Tissue-engineered trachea scaffold materials require satisfactory biomechanical properties in order to fulfill functions of the trachea. The present study evaluated the biomechanical properties of dry and wet NCECS/nHA composites to better understand the effects of nHA on the biomechanical properties of NCECS composites. We found that nHA produced positive mechanical effects on NCECS. It turned out that NCECS/nHA composites exhibited increasing tensile strength and elasticity. Importantly, our results indicate that the mechanical properties of NCECS/nHA correspond to the requirement of the order of magnitude (1.9-14.4 MPa) of normal cartilage [Citation15,Citation16].
Cellular adhesion on material surface is one of the important indices to evaluate the biocompatibility of materials. The present study seeded tracheal chondrocytes onto the surface of NCECS/nHA composites to observe changes in cell morphology and adhesion and to gain insight into the biocompatible nature of NCECS/nHA. The results showed that the cell adhesion rate after 2 and 6 hours of culture was significantly greater than that in the control group. The low early-stage cell adhesion rate potentially relates to the hydrophobicity of the composite material. How to further increase the hydrophilicity of NCECS/nHA for better early-stage cell adhesion and growth is important for optimizing the biocompatibility of NCECS/nHA. The mitochondrion is one of the most sensitive indices to detect cell injury. MTT assays are increasingly used to evaluate the biocompatibility of biomaterials. In this present study, tracheal chondrocytes exhibited an S-shaped in vitro growth curve, corresponding to the growth rule of chondrocytes. Notably, the tracheal chondrocytes showed higher proliferative activity in the NCECS/nHA group than in the NCECS and control groups. Scanning electron microscopy results also demonstrated that cells in the NCECS/nHA group exhibited high cell density, regular cell morphology, and good extensibility.
In conclusion, the three-dimensional porous NCECS/nHA composite materials prepared by a freeze-drying method can overcome the disadvantages of chitosan, including rapid degradation in the physiological environment and low mechanical strength, as well as compensate for the shortcomings of HA including granular fluidity and poor membrane formation. The composite material provides good porosity and satisfactory biomechanical properties and is thus promising for potential construction of tissue-engineered trachea.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Grillo, H.C. (2002). Tracheal replacement: A critical review. Ann Thorac Surg 73:1995–2004.
- Sun, T.S., Guan, K., Shi, S.S., Zhu, B., Zheng, Y.J., Cui, F.Z., Zhang, W., Liao, S.S. (2004). Effect of nano-hydroxyapatite/collagen composite and bone morphogenetic protein-2 on lumbar intertransverse fusion in rabbits. Chin J Traumatol 7:18–24.
- Liuyun, J., Yubao, L., Chengdong, X. (2009). Preparation and biological properties of a novel composite scaffold of nano-hydroxyapatite/chitosan/carboxymethyl cellulose for bone tissue engineering. J Biomed Sci 14(16): 65.
- Peter, M., Ganesh, N., Selvamurugan, N., Nair, S.V., Furuike, T., Tamura, H., Jayakumar, R. (2010). Preparation and characterization of chitosan-gelatin/nano hydroxyapatite composite scaffolds for tissue engineering applications. Carbohydrate Polymers 80:687–694.
- Cook, S.D., Thomas, K.A., Dalton, J.E., Volkman, T.K., Whitecloud, T.S. 3rd, Kay, J.F. (1992). Hydroxyapatite coating of porous implants improves bone ingrowth and interface attachment strength. J Biomed Mater Res, 26(8): 989–1001.
- Teng, S.H., Lee, E.J., Wang, P., Shin, D.S., Kim, H.E. (2008). Three-layered membranes of collagen/hydroxyapatite and chitosan for guided bone regeneration. Biomed Mater Res 87B:132–138.
- Teng, S.H., Lee, E.J., Wang, P., Shin, D.S., Kim, H.E. (2008). Three-layered membranes of collagen/hydroxyapatite and chitosan for guided bone regeneration. J Biomed Mater Res B Appl Biomater, 87(1):132–138.
- Khor, E., Lim, L.Y. (2003). Implantable application of chitin and chitosan. Biomaterials, 24(13):2339–2349.
- Jung, U.W., Song, K.Y., Kim, C.S., Lee, Y.K., Cho, K.S., Kim, C.K., Choi, S.H. (2007). Effects of a chitosan membrane coated with polylactic and polyglycolic acid on bone regeneration in a rat calvarial defect. Biomed Mater, 2(3):S101–105.
- Seol, Y.J., Lee, J.Y., Park, Y.J., Lee, Y.M., Young, K., Rhyu, I.C., Lee, S.J., Han, S.B., Chung, C.P. (2004). Chitosan sponges as tissue engineering scaffolds for bone formation. Biotechnology Letters, 26(13):1037–1041.
- Chen, Y., Duo, Y.Q., Luo, Y.J., Tan, H.M. (2004). Preparation and characterization of 3, 4, 5-trimethoxy benzoylate chitosan. Polymeric Materials Science and Engineering, 20(3):210–212 (in Chinese).
- Kong, S.L., Wang, A.Q. (2006). Synthesis and adsorption properties of N, O-carboxyethyl-chitosan. Polymeric Materials Science and Engineering, 22(3):25–29 (in Chinese).
- Pan, Y., Luo, X., Zhu, A., Dai, S. (2009). Synthesis and physicochemical properties of biocompatible N-carboxyethylchitosan. J Biomater Sci Polym Ed, 20(7–8): 981–992.
- Jayakumar, R., Prabaharan, M., Nair, S.V., Tokura, S., Tamura, H., Selvamurugan, N. (2010). Novel carboxymethyl derivatives of chitin and chitosan materials and their biomedical applications. Progress in Materials Science 55: 675–709.
- Gu, Z.Q., Xiao, J.M., Zhang, X.H. (1998). The development of artificial articular cartilage-PVA-hydrogel. Biomed Mater Eng, 8(2):75–81.
- Magnussen, R.A., Guilak, F., Vail, T.P. (2005). Cartilage degeneration in post-collapse cases of osteonecrosis of the human femoral head: Altered mechanical properties in tension, compression, and shear. J Orthop Res, 23(3): 576–583.