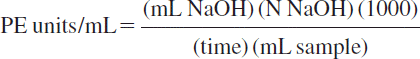Abstract
Abstract: Pectinesterase isolated from Malatya apricot pulp was noncovalently and covalently immobilized onto bentonite and glutaraldehyde-containing amino group functionalized porous glass beads surface at pH 8.0 and pH 9.0, respectively. The effect of various parameters such as pH, temperature, activation energy, heat and storage stability on immobilized enzyme were investigated. The optimum temperature of covalently and noncovalently immobilized PE was 50°C. This value was 60°C for free PE. Although optimum pH of covalently-immobilized PE was 8.0, this parameter was 9.0 for free and covalently-immobilized PE. The noncovalently immobilized enzyme exhibited better thermostability than the free and covalently immobilized PE.
INTRODUCTION
In recent years, immobilization technology involving covalent and/or noncovalent fixation of enzymes, drugs, and proteins on soluble or insoluble polymeric cariers has received considerable interest. Biocatalysts immobilized on organic or inorganic polymer supports help in their economic reuse and in the development of continuous bioprocesses. Biocatalysts can be immobilized either using the isolated enzymes or the whole cells. Immobilization often stabilizes the structure of the enzymes, thereby allowing their application even under harsh environmental conditions of pH, temperature, and organic solvents, and thus enables their use at high temperatures in nonaqueous enzymology and in the fabrication of biosensor probes.
There are various methods for immobilization of enzymes and these may be divided into physical methods based on molecular interactions between the enzyme and carrier, and chemical methods based on formation of covalent bonds [Citation1]. The success of an immobilized enzyme for practical applications depends strongly on the properties of the carriers employed. The carrier material must be insoluble in water, should have a high capacity to bind enzyme, be mechanically stable, and must not have a deleterious effect on the enzyme action. Various modified supports for covalent immobilization such as polymers, silica, glass, and soils have been widely investigated [Citation2].
Polymers, silica, and glass as modified supports for covalent immobilization have been widely investigated. Silica gel, aluminum oxide, apatite, and glass supports are also preferred inorganic support materials. Inorganic carriers employed in biotechnology are usually modified bifunctional organosilanes; in particular, 3-aminopropyltriethoxysilane. Such modified carriers, especially porous glasses having controlled pore dimensions, are widely applied in biotechnology, predominantly for enzyme immobilization. The hydroxyl groups on the surface of silica and glass provide desired functionality that can react with hydrolysable groups of organosilane and cyanogens bromide coupling agents. Silanisation technique is used to change the physical and chemical properties of solid hydrophilic surface properties of silicates and glass [Citation3].
Although polymeric systems have largely been employed as carriers of immobilized enzymes, a great deal of work has also been done on the enzyme-soil interaction, which is also an important natural phenomenon in terrestrial and ecosystems [Citation4, Citation5]. Thus, the adsorption of proteins and enzymes onto mineral surfaces is a subject of great significance and, therefore, deserves more attention. Realizing the need for a careful investigation of enzyme-mineral interaction, the proposed study involves to undertake the immobilization of pectin esterase onto bentonite mineral surfaces.
Bentonites possess important and unique properties which give them great commercial value in decolorizing oils, in the manufacture of catalysts, in bonding of molding sands, in the preparation of oil-well drilling muds, and in many other relatively minor uses. It has also widespread use for clarification of edible and mineral oils, paints, cosmetics, and pharmaceuticals [Citation5]. It has been considered a good candidate matrix for enzyme immobilization since it exists naturally in the immobilized form in soil and participates in humification processes. In addition, it has the highest absorbent capacity of any mineral clay, as well as a high adsorptive capacity, because it has high surface area [Citation6].
Pectolytic enzymes play a crucial role in food processing industries, e.g., in the production of fruit juices, soft drinks, and liquors. These enzymes are also used in the maceration, liquefaction, and extraction of vegetable tissues. They also help to reduce the viscosity of fruit pulp which, in turn, improves filtration and clarification of fruit juices and wood aging. Therefore the immobilization of these enzymes is very important for their use at these industrial areas [Citation2]. The enzyme pectinesterase (PE, EC 3.1.1.11) has been found in plants as well as in pathogenic fungi and bacteria and catalyzes the hydrolytic cleavage of the methylester moieties on pectin molecules, resulting in the release of methanol and partially de-esterified pectin.
PE is of significance to the citrus industry since it has been definitively established as the causative agent of juice clarification and gelation of frozen concentrates. Pectinesterase (PE) is well described in higher plants. It has been extracted and/or purified from different sources including acerola [Citation7], pear [Citation8], soursop fruits [Citation9, Citation10], lemon [Citation11], tomatoes [Citation12–14], oranges and grapefruits [Citation15, Citation16], apples [Citation17, Citation18], and bananas [Citation19]. The properties of PE obtained from different fruits vary; therefore, it is advisable to determine the optimum conditions for the processing of different fruits [Citation20].
The determination of PE activity has been reported as a way to obtain information about the degree of fruit juices and concentrates stability [Citation15, Citation21–23]. The PE assays are commonly based on spectrophotometry using chemical [Citation15, Citation24] or enzymic derivatisation [Citation25, Citation26], or on spectrofluorimetry with enzymic derivatisation [Citation27].
In this study, the covalently and noncovalently immobilizations of pectinesterase isolated from Malatya apricot were carried out. Bentonite and porous glass beads were used as immobilization matrix materials. The characterizations of the immobilized enzymes were carried out by determining kinetic constants, optimum pH and temperature, activation energy, thermal and storage stability, and reusability. The properties of the porous glass beads- and bentonite-immobilized PE enzyme were compared with those of the free enzyme.
EXPERIMENTAL
Materials
Pectinesterase enzyme used in this work was isolated from apricot harvasted from the Malatya region in Turkey, purchased at commercial maturity from a local market, and stored at −20 °C. Bentonite used in this study was obtained from MTA (Ankara, Turkey). Porous glass beads for enzyme immobilization, membranes cut off 12000 (Sigma Chem. Co., St. Louis, MO) used for dialysis, pectin, KH2PO4 (99,0%) were purchased from Sigma Chem. Co. (St. Louis, MO); (3-aminopropyl) triethoxy silane (APTS), (NH4)2 SO4, Na2HPO4 and H2SO4 were purchased from Fluka (Riedel-de Haën, Sigma-Aldrich Laborchemikalien, Seelze, Germany); sodium azid, potassium tartarate, sodium tungstate, sodium molybdate, CuSO4.5H2O, Li2SO4, NaOH, HCI, NaCI were purchased from Merck (Schuchardt OHG, Hohenbrunn, Germany). All chemicals used in this study were of analytical grade and were used without further purification.
Spectrophotometric measurements were carried out with a Shimadzu UV-Vis-1601 spectrophotometer. pH measurements were carried out with an Orion 720A model pH-meter, Centrifugation was made by using the Beckman Optima XL-100K model; the measurements related to temperature were carried out with a mixed and thermostated water bath (Thermolyne Cimarca), and the mixing procedure was made by using a vortex (Nüve N 110).
Methods
Isolation and Partial Purification of Malatya Apricot Pectinesterase. Pectine esterase was isolated and characterized at the previous work [Citation28]. 500 g Malatya apricots were stored at −20°C during one week and kept at +4°C for dissolving the frosted fruit. Then, the apricots were halved, cored, and weighed (465 g.). The apricot tissue was homogenized with 931 mL of 2N NaCI using a Waring blender for 5 min and the homogenate was filtered with cheesecloth. The homogenate, called a crude enzyme extract, was centrifuged at 24.000 × g for 30 min at 4°C. The enzyme solution from the supernatant was fractionated with solid ammonium sulfate and the precipitate of 40–80% saturation was collected by centrifugation at 24.000 × g for 30 min at 4°C. For the ammonium sulfate precipitation, the addition of solid ammonium sulfate was carried out slowly by completely dissolving it after each addition during one hour. The pellet was redissolved in 120 ml homogenization buffer (0.2 M sodium phosphate buffer, pH 7.5) and dialyzed at 4°C against the same buffer in cellulose dialysis tubing (mol. wt. cut off 12,000–14,000 Da). The dialysis buffer was changed five times with 8-hr intervals. The dialyzed solution was kept in stoppered test tubes at −20°C. The partially purified enzyme was used for the characterization of apricot pectinesterase enzyme because it showed higher activity.
Immobilization Procedure
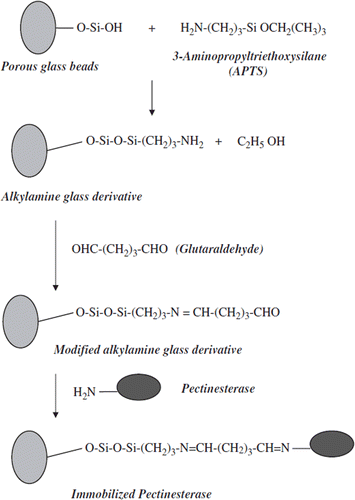
Covalent Immobilization. The covalent immobilization of PE isolated from Malatya apricot was carried out at our previous study [Citation3]. First of all, porous glass beads were activated by (3-aminopropyl) triethoxy silane (APTS) for immobilization of the PE enzyme isolated from Malatya apricot (Prunus armeniaca L.) on porous glass beads. The silanization procedure was performed as described by [Citation29]. The α-amino groups of lysine on enzyme molecules were attached to the aminopropyl glass beads via glutaraldehyde (2.5%) (). 6.0 g glass beads were first washed several times with distilled water and then suspended in 20 ml of 10% APTS solution for silanization process. The pH of the medium was adjusted between pH 3.0–4.0 with concentrated HCl and then was refluxed gently (150 rpm) via magnetic stirrer at 75°C for 5 hours. The glass beads activated with APTS were washed with distilled water for three times. The aminopropyl-glass derivative was suspended in 150 ml of 2.5% glutaraldehyde solution (prepared in sodium phosphate buffer, 0.2 M, pH 7.5) and was agitated at 200 rpm at room temperature for one hour. The beads were separated and washed with the same buffer. They were added to 10 ml of partially purified pectinesterase enzyme isolated from Malatya apricot, which was prepared in a 0.2 M sodium phosphate buffer (pH 7.5) with gentle shaking and incubated at 4°C overnight with a magnetic stirrer. The immobilized enzyme conjugate was centrifugated at 12,000 rpm during 15 min. and then, in order to remove unbound proteins, it was washed with the same buffer and dried at 4°C. For each centrifugation procedure the supernatant was analyzed for remaining pectinesterase activity by our modified method determined by [Citation30] as described above.
Noncovalent Immobilization. PE isolated from Malatya apricot was noncovalently immobilized at a previous study [Citation31]. Before bentonite is used to immobilize the pectinesterase enzyme, it was mixed in 50 ml 3M H2SO4 for 2 h, filtered and washed several times with distilled water, then dried at room temperature for a week. 5.0 g of acid-treated bentonite was suspended in 15 ml of pectinesterase isolated from Malatya apricot (Prunus armeniaca L.) after it was diluted at a 1:1 ratio with 0.2 N sodium phosphate buffer (pH 7.5) solution. After mildly stirring for 90 min at 4°C, the suspension was centrifuged at 12,000 rpm at room temperature for 15 min and the precipitate was washed three times with 0.2 N sodium phosphate buffer solution (pH 7.5). For each centrifugation procedure, the supernatant was analyzed for remaining pectinesterase activity by using our modified method proposed by [Citation30] as described above.
Protein Assay
The protein assay of the each supernatant was carried out by the Bradford protein assay method [Citation32]. The amount of adsorbed protein was calculated from the difference between the amount of protein introduced into the reaction mixture and the amount of protein in the filtrate and washing solutions after immobilization.
Determination of Enzyme Activity
Pectinesterase activity was determined manually by our modified method proposed by [Citation30]. Briefly, the method involves the measurement of the releasing rate of carboxyl groups in a pectin solution in 0.1 N NaCl (1% w/v) at room temperature. Pectin (1%) in 0.1 N NaCl (1% w/v) was prepared and stored according to a procedure described by [Citation33]. The reaction was started by the addition of 0.05 g immobilized PE dissolved in 0.5 mL sodium phosphate buffer (pH 7.5) enzyme to 4 ml of 1% pectin solution in 0.1 N NaCl (1% w/v) and the reaction pH was adjusted and maintained manually at the optimum pH of the enzyme by the addition of 0.1 and/or 0.01 N NaOH solution. After the pH was fixed, 0.2 ml 0,01 N NaOH was added and provided a sudden increase in pH. The returning “time” to adjusted pH from suddenly increased pH is recorded. PE activity was calculated both immobilized enzymes by the following formula [Citation11, Citation13, Citation34]:
The method was also carried out at various temperature and pH values with pectin substrate for characterization of the enzyme. One unit of PE activity was defined as the amount of enzyme that released 1 μmol of carboxyl group produced in min. at room temperature. Experiments were triplicated.
Effect of pH on Immobilized Enzyme Activity
The effect of pH on free and immobilized pectinesterase enzymes activities were assayed at different pH values between 4.0 and 9.5. 0.05 g enzyme dissolved with 0.5 mL 0.2 M phosphate buffer (pH 7.5) was added into 4 mL 1% pectin in 0.2 N NaCl. The pH influence was studied manually with 0.2 mL 0.01 N NaOH after adjusting the pH of the reaction solution to one of the pH values (4.0–9.5) tested. The optimum pH value obtained from these assays was used in all the other experiments.
Effect of Temperature on PE Activity
The effect of temperature on free enzyme and immobilized enzymes was tested manually with 1% pectin concentration at pH 9.0 and PE activity as a function of temperature under standard assay conditions was determined by using temperatures from 10 to 90°C controlled by means of a circulating water bath. To determine relative activity of PE at a specific temperature, 0.05 g enzyme dissolved with 0.5 mL 0.2 M phosphate buffer (pH 7.5) was incubated in 15 min in a circulating water bath, cooled, and added to 1% pectin.
Thermal Stability of Apricot PE
Thermal stability studies of free and immobilized pectinesterase enzymes were performed between 60 and 90°C temperatures manually under standard assay conditions by measuring the residual activity of the enzyme in a 0.2 M sodium phosphate buffer (pH 7.5). 0.05 g enzyme dissolved with 0.5 mL 0.2 M sodium phosphate buffer (pH 7.5) in three different test tubes was incubated in 45 min. in a circulating water bath at the specified temperature Enzyme was withdrawn from each tube at at various time intervals during 45 min. After being cooled, it was added into 1% pectin for various time intervals (15–45 min). The remaining activity of the enzyme was determined.
Determination of Activation Energy (Ea)
Activation energy (Ea) of free and immobilized Malatya apricot PEs was estimated from the slope of the Arrhenius plot obtained by plotting the logarithm of reaction rate constants (ln k) vs. the reciprocal of the absolute temperature (1/T). Thermal inactivation rate constants were calculated by comparing the activity changes upon heat treatment with the unheated enzyme extracts as reported by [Citation35].
Kinetic Constants of Apricot PE
Determination of Km and Vmax values of free and immobilized enzymes was carried out by measuring pectinesterase activities in the presence of various substrate concentrations (0.05, 0.075, 0.125, 0.25, 0.5, 0.75, 1 mM). Michael-Menten constant (Km) values and the maximum velocities (Vmax) were determined using the Lineweaver–Burk double reciprocal plot [Citation36], in which the reciprocals of the initial velocities of the pectinesterase activity were plotted against the reciprocals of the concentration of pectin used.
RESULTS AND DISCUSSION
Extraction and Partial Purification of PE
PE is of significance to the fruit juice industry since it has been definitively established as the causative agent of juice clarification and gelation of frozen concentrates. The enzyme catalyzes the hydrolytic cleavage of the methylester moieties on pectin molecules, resulting in the release of methanol and partially de-esterified pectin [Citation37].
Initially, the isolation and partially purification of pectinesterase enzyme from Malatya apricot pulp were carried out by using 2 M NaCl prepared with a 0.2 M sodium phosphate buffer (pH 7.5). For the isolation and partially purification procedure, it was carried out homogenization with NaCl, ultrafiltration at 4°C, saturation with ammonium sulphate and dialysis, respectively. The optimum concentration of NaCl used for adjusting ionic strength of the solution in this study was similar to that found by us in extracting pectinesterase from apricot pulp [Citation38]. The precipitate obtained from 40–80% ammonium sulphate fractionation of crude extract of 420 g of apricot pulp dissolved and dialysed with 0.2 M sodium phosphate buffer (pH 7.5). In , it was seen that 5.2-fold and 12.2-fold purification were obtained with 80% saturation of solid ammonium sulphate and dialysis, respectively. Dialysis membrane is used to remove salts and low-molecularweight-inhibitor(s) used in isolation and purification procedures of enzyme solution. Immobilization procedure was carried out with the dialysed enzyme named a partially purified enzyme.
Table 1. Purification of pectinesterase enzyme isolated from Malatya apricot.
Immobilization of PE from Malatya Apricot
Pectinesterase enzyme isolated from Malatya apricot pulp was covalently immobilized on commercially available inexpensive and renewable porous glass beads. The covalent immobilization method requires chemical modification of the glass surface, so that functionally inert silanols (Si–OH) of a glass surface are modified to posses either nucleophilic or electrophilic functionalities that react with the enzyme reactive functional groups (–NH2). Inorganic carriers employed in biotechnology are usually modified bifunctional organosilanes: in particular, 3-aminopropyltriethoxysilane. Such modified carriers, especially porous glasses having controlled pore dimensions, are widely applied in biotechnology, predominantly for enzyme immobilization [Citation39].
After surface modification of the glass beads was carried out with a silane-coupling agent (3-aminopropyl triethoxysilane), enzyme immobilization was achieved. After this modification procedure, the surface modified glass beads were reacted with glutaraldehyde followed by covalent attachment of the PE enzyme to the newly introduced functional group on the surface. The procedure used for covalently enzyme immobilization on the activated glass bead surface is shown in . The immobilization results are summarized in .
Table 2. Immobilization of PE from Malatya apricot on porous glass beads.
In the second study, partially purified PE enzyme isolated from Malatya apricot pulp was immobilized on bentonite by adsorption. Bentonite is an attractive enzyme immobilization material since the immobilization of enzyme on this support is easy and inexpensive. Furthermore, this method is a very economical procedure for the immobilization of enzymes. The stability of the adsorbed enzyme derivative will depend on the strength of the noncovalent bonds formed between the support and the amino acid residues on the surface of the protein. Two principal types of bonds can be formed between the enzyme and the inorganic support: electrostatic bonds and hydrogen bonds. The main disadvantage of this method is the weak binding of enzymes [Citation3].
The amount of covalently and noncovalently bound apricot PE was found 1.721 mg/g glass support and 1.141 mg/g bentonite, respectively. Increasing the amount of enzyme caused a decrease in activity ( and ). This has been explained by “crowding” of the enzyme on the surface of bentonite for noncovalent immobilization.
Table 3. Inactivation rate constants and of free and immobilized apricot PEs.
After enzyme isolation and immobilization studies, some properties of the immobilized enzymes were investigated and also demonstrated and compared with the free enzyme.
Effect of pH on PE Activity
The activity of free and immobilized pectinesterases were studied within the pH range of 4.0–9.5 at room temperature. The enzyme activities obtained are presented in .
Figure 2. The effect of pH on the activity of free and immobilized apricot PE. Reactions were made with 0.5 mL 0.2 M phosphate buffer (pH 7.5) into 4 mL 1% pectin in 0.2 N NaCl.
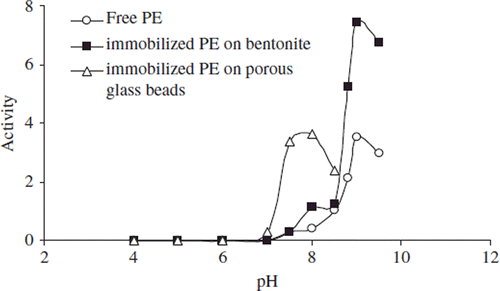
The maximum activity was observed at pH 9.0 for free and bentonite-immobilized pectinesterase while those of the immobilized enzyme onto porous glass beads was towards 1.0 pH unit to the acidic region. This is explained in that secondary interactions, such as ionic and polar interactions, hydrogen bonding between the enzyme and the support matrix, possibly occurred [Citation40]. Because of the amino groups occurred after silanization, pH values changes to acidic region. Strong interactions between enzyme and support will affect the intra-molecular forces responsible for maintaining the conformation of the enzyme that would lead to a change activity. The polar groups of porous glass beads may have interacted with the functional groups of pectinesterase changing the pH characteristics of the enzyme. The change depends on the enzyme reaction as well as on the structure and the charge of the matrix. In the optimum pH of α-amylase immobilized on glass beads, it changed towards the acidic direction, which was observed [Citation41]. In Reference [Citation42] it was determined that the optimum pH of immobilized phospholipase A2 enzyme on porous glass beads was 8.5. There was no shift from the pH optima of the free enzyme.
Effect of Temperature on PE Activity
The activities of free and immobilized pectinesterases were assayed at various temperatures (10–90°C). The effect of temperature on the activity of free and immobilized apricot PE is shown in .
Figure 3. The effect of temperature on the activity of free and immobilized apricot PE. Reactions were made with 1% pectin concentration at pH 9.0.
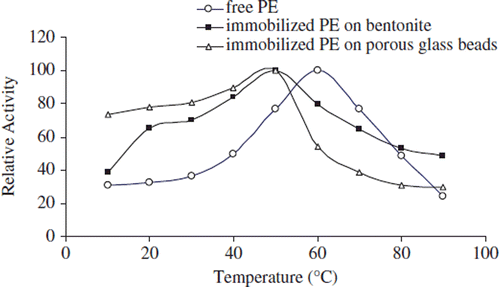
The maximum catalytic activity was obtained at 60°C for the free enzyme and 50°C for both immobilized enzymes. After optimum temperature, the stability of the free enzyme reduces rapidly compared to those of the immobilized form against the temperature increases. Although the optimum temperature of the free enzyme is bigger than those of the immobilized enzymes, immobilized enzymes are importantly more thermostable than free ones. The immobilization procedure probably helps to maintain the oligomeric forms (mainly octameric and hexameric aggregates) of the enzyme prevailing in the free pectinesterase enzyme [Citation43]. The stability of immobilized pectinesterase is significantly improved over that of the free form at lower and higher temperatures. The mobility of the pectinesterase molecules probably increases with increasing temperature since the immobilization is normally a diffusion-controlled process. This situation can cause increased immobilization efficiency. At higher temperature, the electrostatic attractions are normally weakened and, therefore, a lower immobilization should be expected [Citation44].
Thermal Stability of Free and Immobilized Apricot PE
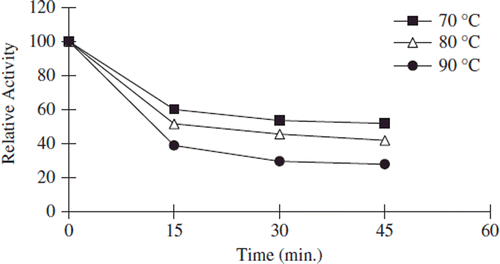
Thermal stability of free and immobilized enzyme was determined with the incubation of samples in the absence of substrate at various temperatures. After its immobilization on a support, thermal stability of many enzyme was increased because the support material is supposed to preserve the tertiary structure of the enzyme. In addition, it is pointed out that the thermal stability of an enzyme may indicate the efficiency of the immobilization method and reflect the delicate balance between the acquired conformational stability and the resulting microenvironment created around the enzyme [Citation45]. The authors demonstrated that the thermal stability of enzymes might be drastically increased if they are attached to a complementary surface of a relatively rigid support in a multipoint [Citation17]. , , and show the heat inactivation curves between 70 and 90°C for free, covalently and noncovalently immobilized pectinesterases, respectively. Free and immobilized enzymes retained 30%, 40%, and 45% activity, respectively, at 70°C. At 90°C, during 45 min of incubation period, the lost activity of free and immobilized pectinesterase enzymes was about 95%, 80%, and 55% for 45 min, respectively. Free enzymes lost on a large scale their initial activity at 90°C after 45 min. At all temperatures, the bentonite-immobilized enzyme was inactivated at a much slower rate than the free and covalently immobilized forms.
Figure 4. The thermal stability profıle for free apricot PE. Reactions were made with 0.5 mL enzyme in 0.2 M phosphate buffer (pH 7.5) into 4 mL 1% pectin in 0.2 N NaCl at pH 9.0.
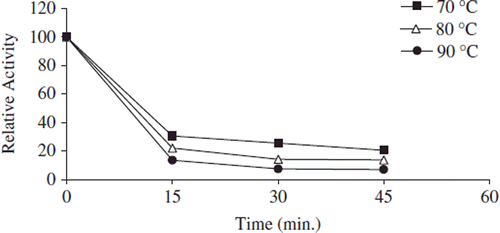
Figure 5. The thermal stability profıle for covalently immobilized apricot PE. Reactions were made with 1% pectin concentration and 0.05 g immobilized enzyme dissolved with 0.5 mL 0.2 M sodium phosphate buffer (pH 7.5) at pH 9.0.
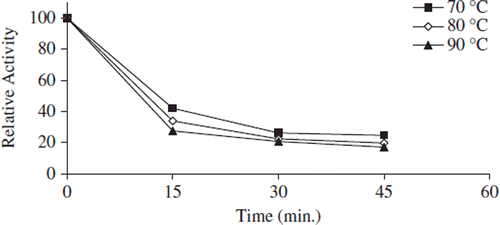
Figure 6. The thermal stability profıle for noncovalently immobilized apricot PE. Reactions were made with 1% pectin concentration and 0.05 g immobilized enzyme dissolved with 0.5 mL 0.2 M sodium phosphate buffer (pH 7.5) at pH 9.0.
The data obtained from the thermal stability profile were used to analyze some thermodynamic parameters related to apricot PE activity. The thermal inactivation rate constants k were calculated from the slope of the linear part of the curve at each temperature for free and immobilized PEs. The temperature dependence of k was evaluated using the Arrhenius equation. From a plot of ln k–1/T, Ea (activation energy) was calculated from the slope of the straight line and found to be 2.96 kcal mol−1 for free enzyme, 5.03 kcal mol−1 for noncovalently immobilized PE, and 0.9 kcal mol−1 for covalently immobilized PE.
The thermal inactivation rate constants (k) for free and immobilized enzymes were determined from the percentage residual activity versus time, at three different temperatures; the results are presented in . The half-life of the noncovalently immobilized PE is bigger than free and covalently immobilized PE (). Inactivation rate constants (k) for free and covalently and noncovalently immobilized PEs at 70°C were 2.55 × 10−3, 2.46 × 10−3 and 1.55 × 10−3 min−1, respectively. The inactivation rate constant of the free enzyme is bigger than those of noncovalently immobilized PEs while the half-life of the immobilized PE is longer than free ones.
Because the inactivation rate constant is higher, the thermostability of an enzyme is less [Citation46]. The thermostability of the free enzyme is lower than noncovalently and covalently immobilized PEs. These results show that the thermostability of the immobilized enzyme increased after its noncovalent immobilization on bentonite. The activity of the immobilized enzyme, especially in a noncovalently bound system, is more resistant to heat and denaturing agents than that of the free and covalently bound forms. If the thermal stability of an enzyme was increased by immobilization, the potential utility of such enzymes would be extensive [Citation40].
Enzyme Kinetic Studies
The kinetic constants (Km and Vmax values) for free and immobilized pectinesterase enzymes were determined by using pectin as a substrate (). Km and Vmax values for the free and immobilized PE were calculated from the intercepts on x and y axes of the Lineweaver- Burk plots [Citation36]. For the free enzyme Km was 0.77 mM and the apparent Km value of covalently immobilized and noncovalently pectinesterase was 0.71 mM and 0,51 mM, respectively. Km values for free and immobilized PE enzymes were almost equal. For the free enzyme Vmax was 1.75 U μmol min−1mg−1, but upon covalent immobilization of the enzyme on porous glass beads Vmax decreased to 0.64 μmol min−1mg−1. It may be possible that immobilization of the enzyme also occurred inside the porous space of glass beads, thus increasing mass transfer resistance of substrate and product. During the covalent immobilization, structural changes in the enzyme molecule procedure can occur and cause the change in the kinetic parameters of the immobilized enzyme [Citation3].
Table 4. Kinetic properties of free and covalently and noncovalently-immobilized apricot PEs.
The Vmax value of bentonite-immobilized PE enzyme is importantly increased as 14,6 μmol min−1mg−1. The results indicated that the adsorption of pectinesterase enzyme enables it to react more quickly. Reference [Citation47] calculated the value of Km of 13.90 mM and the Vmax of 65.78 μM/min for free catalase; the Km of 13.22 mM and the Vmax of 55.86 μM/min were stated for the bentonite-immobilized catalase.
Storage Stability Studies
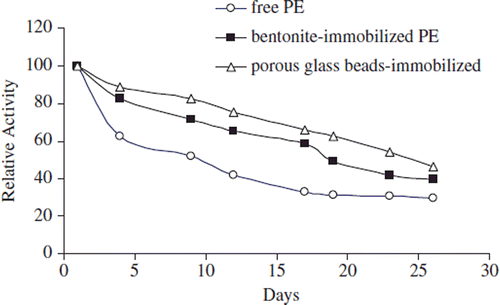
For the studies of storage stability of free and immobilized enzymes, the enzyme preparations were stored at 4°C and measured for a period of 30 days. Results are shown in . During the first five days, the activity lost of 40%, 10%, and 15% for free and bentonite- and porous glass beads-immobilized apricot PE enzymes were observed, respectively. Activities were decreased slowly after the ten days and continued to decrease until day 30 ().
The free enzyme lost about 70% of all its initial activity while the immobilized enzymes retained about 50% for bentonite–immobilized PE and 40% for glass beads–immobilized PE of its initial activity after 30 days (). The porous glass beads and the immobilization method provide higher shelf-life compared to that of the free enzyme and bentonite-immobilized enzyme since the covalent bonds formed between the enzyme and support enhance the conformational stability of the immobilized enzyme.
CONCLUSION
In this study, covalent and noncovalent immobilizations of pectineesterase isolated from Malatya apricot (Prunus armeniaca L.) were carried out. The immobilization procedures proposed were very easy to carry out. The immobilized enzymes also showed good properties that are very important parameters in applications. Optimum pH was observed at pH 9.0 for free and bentonite-immobilized pectinesterase while those of the immobilized enzyme onto porous glass beads was pH 8.0. The optimal temperatures for free, covalently and non-covalently immobilized PEs were 60, 50, and 50°C, respectively. The thermal stability of the enzyme was increased by noncovalent immobilization. The thermostability of the free enzyme was lower than noncovalently and covalently immobilized PEs. These results show that the thermostability of the immobilized enzyme increased after its noncovalent immobilization on bentonite. The activity of the immobilized enzyme, especially in a noncovalently bound system, was more resistant to heat and denaturing agents than that of the free and covalently bound form.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Hartmier, W., Immobilized Biocatalysts, An Introduction, Springer Verlag, Berlin, 1988.
- Reshmi, R., Sanjay, G. and Sugunan, S. (2007). Enhanced activity and stability of α-amylase immobilized on alumina. Catalysis Communications 8:393–399.
- Karakuş, E. and Pekyardımcı, Ş. (2008). Immobilization of apricot pectinesterase (Prunus armeniaca L.) on porous glass beads and its characterization. Journal Of Molecular Catalysis B-Enzymatic 56:13–19.
- Moustafa, A.B., Kahil, T. and Faizalla, A. (2000). Purification and biochemical characteristics of pectinesterase from Malatya apricot (Prunus armeniaca L.). J App. Polym Sci 76:594.
- Takami, H., Abika, T. and Horikoshi K. (1989). Production of extremely thermostable alkaline protease from Bacillus sp. no.AH-101. Appl Microbiol Biotechnol 30:120–124.
- Yağar, H. and Sağiroğlu, A. (2002). Non-Covalent Immobilization of Quince (Cydonia Oblonga) Polyphenol Oxidase. Turk J Chem 26:751–758.
- Assis, S.A., Martins, A.B.G. and Oliveira, O.M.M. (2007). Purification and characterization of pectin methylesterase from acerola (Malpighia glabra L.). Journal of the Science of Food and Agriculture 87:1845–1849.
- Contreras-Esquive, J.C., Correa-Robles, C., Aguilar, C.N., Rodriguez, J., Romeroc, J. and Hoursd, R.A. (1999). Pectinesterase extraction from Mexican lime (Citrus aurantifolia Swingle) and prickly pear (Opuntia ®cus indica L.) peels. Food Chemistry 65:153–156.
- Arbaisah, S.M., Asbi, B.A., Junainab, A.H. and Jamilah, B. (1996). Determination of optimum conditions for pectinesterase extraction from soursop fruit (Anona muricata) using response surface methodology. Food Chemistry 55:289–292.
- Arbaisah, S.M., Asbi, B.A., Junainab, A.H. and Jamilah B. (1997). Purification and properties of pectinesterase from soursop (Anona muricata) pulp. Food Chemistry 59:3340.
- MacDonald, H.M., Evans, R.E. and Spencer, W.J. (1993). Purification and properties of the major pectinesterase in lemon fruits (citrus lemon). J Sci Food Agric 62:163–168.
- Whitaker, J.R. (1984). Pectic substances, pectic enzymes and haze formation in fruit juices. Enzyme Microb Technol 6:341–349.
- Giovane, A.L., Quagliuolo, L., Servillo, C., Balestrieri, B., Laratta, R., Loiudice, D., . (1994). Purification and characterization of three isozymes of pectin methylesterase from tomato fruit. J Food Biochem 17:339–349.
- Tijskens, L.M.M., Rodis, P.S., Hertog, M.L.A.T.M., Proxenia, N. and Van Dijk, C. (1999). Activity of pectin methyl esterase during blanching of peaches. J Food Eng 39:167–177.
- Seymour, T.A., Preston, J.F., Wicker, L., Lindsay, J.A. and Marshall, M.R. (1991). Purification and properties of pectinesterase of marsh white grapefruit pulp. J Agric Food Chem 39:1080–1085.
- Ingallinera, B., Barbagallo, R.N., Spagna, G., Palmeri, R. and Todaro, A. (2005). Effects of thermal treatments on pectinesterase activity determined in blood oranges juices. Enzyme and Microbial Technology 36:258–263.
- Denes, J.M., Baron, A.A. and Drilleau, J.F. (2000). Purification, properties and heat inactivation of pectin methylesterase from apple (cv Golden Delicious). J Sci Food Agric 80:1503–1509.
- King K. (1990). Partial characterization of the in situ activity of pectinesterase in Bramley apple. Int J Food Sci Technol 25:188–197.
- Brady C.J. (1976). The pectinesterase of the pulp of the banana fruit. Aust J Plant Physiol 3:163–172.
- Fayyaz, A., Asbi, B.A., Ghazali, H.M., Che Man, Y.B. and Jinap, S. (1995). Kinetics of papaya pectinesterase. Food Chem 53:125–129.
- Lin, T.P., Liu, C.C., Chen, S.W. and Wang, W.Y. (1989). Purification and characterization of pectinmethylesterse from Ficus awkeotsang makino achenes. Plant Physiol 91:1445.
- Giovane, A., Quagliuolo, L., Castaldo, D., Servillo, L. and Balestrieri, C. (1990). Pectinmethylesterase from Actinidia chinensis fruits. Phytochem 29:2821–2823.
- Alonso, J., Howell, N., Canet, W. (1997). Purification and characterization of two pectinmethylesterases from persimmon (Diospyros kaki). J Sci Food Agric 75: 352–358.
- Hagerman, A.E. and Austin, P.J. (1986). Continuous spectrophotometric assay for plant pectin methyl esterase. J Agric Food Chem 34:440–444.
- Klavons, J.A. and Bennett, R.D. (1986). Determination of Methanol Using Alcohol Oxidase and Its Application to Methyl Ester Content of Pectins. J Agric Food Chem 34:597–599.
- Mango, J. and Haas, M.J. (1997). A Spectrophotometric Assay for the Enzymatic Demethoxylation of Pectins and the Determination of Pectinesterase Activity. Anal Biochem 244:357–366.
- Wojciechowski, C.L. and Fall, R. (1996). A Continuous Fluorometric Assay for Pectin Methylesterase Anal Biochem 237:103–108.
- Özler, A., Karakuş, E. and Pekyardımcı, Ş. (2008). Purification and biochemical characteristics of pectinesterase from Malatya apricot (Prunus armeniaca L.). Preparative Biochem Biotechnol 38(4):358–375.
- Weethall, H.H. (1993). Preparation of immobilized proteins covalently coupled through silane coupling agents to inorganic supports. Appl. Biochem. Biotechnol. 41:157–188.
- Versteeg, C., Rombouts, K.M., Spaansen, C.H. and Pilnik, W. (1980). Thermostability and orange juice cloud destabilizing properties of multiple pectinesterases from orange. J Food Sci 45:969–971.
- Karaku, E., Özler, A. and Pekyardımcı, Ş. (2008). Noncovalent Immobilization of Pectinesterase (Prunus armeniaca L.) onto Bentonite, Artif Cells Blood Subst and Biotechnol 36 (6):535–550.
- Bradford, M. (1976). A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal Biochem 72:248.
- Rouse A.H. and Atkins C.D. (1955). Preparation of immobilized proteins covalently coupled through silane coupling agents to inorganic supports. Fla Agr Expt Sta Bull 570.
- Delince, H. and Radola, B.J. (1970). Some size and charge properties of tomato pectin methylesterase. Biochem Biophys Acta 214:178–189.
- Amiza, M.A. and Apenten, R.K.O. (1994). Thermal inactivation parameters for alkaline proteinases from North Sea cod (Godus morhua) and bovine α-chymotrypsin. J Sci Food Agric 66:389–391.
- Lineweaver, H. and Burk, D. (1934). The determination of enzyme dissociation constant. J Am Chem Soc 56:658.
- Pilnik, W. and Voragen, A.G.J. (1991) The significance of endogenous and exogenous pectic enzymes in fruit and vegetable processing. In Food Enzymology. P. F. Fox. Elsevier, New York, 1303–36.
- Fayyaz, A., Asbi, B.A., Ghazali, H.M., Che Man, Y.B. and Jinap, S. (1993). Pectinesterase extraction from papaya. Food Chem 49:373–378.
- Halliwell, C.M. and Cass, A.E.G. (2001). A Factorial Analysis of Silanization Conditions for the Immobilization of Oligonucleotides on Glass Surfaces. Anal Chem 73:2476–2483.
- Arica, M.Y. (2000). Immobilization of polyphenol oxidase on carboxymethylcellulose hydrogel beads: preparation and characterization. Polym Int 49:775–781.
- Kahraman, M.V., Bayramoğlu, G., Kayaman-Apohan, N. and Güngör, A. (2007). Alfa-Amylase immobilization on functionalized glass beads by covalent attachment. Food Chem 1041:385–1392.
- Teke, M., Önal, S., Kılınc, A. and Telefoncu, A. (2003). Immobilization of phospholipase A2 on porous glass and its application for lowering serum cholesterol concentration. Artificial Cells Blood Subst and Biotechnol 31(4):467–478.
- Versteeg, C., Rombouts, F.M. and Pilnik, W. (1978). Purification and some characteristics of two pectinesterase isoenzymes from orange. Lebensm Wiss Technol 1:267–274.
- Bajpai, A.K. and Sachdeva, R. (2002). Immobilization of diastase onto acid-treated bentonite clay surfaces. Colloid Polym Sci 280:892–899.
- Warrilow, A.G. and Jones, M.G. (1995). Different forms of tomato pectinesterase have different kinetic properties. Phytochem 39:277–282.
- Marangoni, A.G. (2003). Enzyme kinetics: a modern approach. New Jersey: John Wiley and Sons 140–157.
- Alkan, S., Ceylan, H. and Arslan, O. (2005). Bentonite-supported catalase. J Serb Chem Soc 70(5):721–726.