Abstract
Abstract: Objective: Currently used decellularization procedures have negative effects on extracellular matrix (ECM) integrity. The objective of this study is to evaluate four decellularization methods and their effect on the collagen ultrastructure, mechanical behavior and antigenicity of porcine aortic valves. Methods: Aortic valves were placed in a trypsin, osmotic, trypsin-osmotic or detergent-osmotic solution. Leaflets were processed for histology and mechanical testing. Matrices were implanted subdermally in rats to evaluate immune reaction and calcification. Results: Trypsin-osmotic methodology effected near-complete decellularization. Trypsin treatment resulted in cell removal only in the spongiosa layer. Osmotic and detergent-osmotic treatments did not remove any cells from the cusps. Mechanical strength was significantly inferior in the trypsin (p50,03) and trypsin-osmotic treated group (p50,04). Trypsin and trypsin-osmotic decellularized matrices evoked a strong CD31 inflammatory cell infiltration. Conclusion: Enzymatic-osmotic decellularization appears to be the only effective method to remove cellular components. However, the near cell free scaffolds still evokes a strong CD31 T-cell inflammatory reaction.
INTRODUCTION
Tissue engineered heart valves are a promising therapeutic mode for heart valve replacement. Recently, it has been found that acellular tissue scaffolds provide natural ultrastructural, mechanical, and compositional cues for recellularization and tissue remodeling. The development of new technologies and improvement of existing decellularization methods for biological tissue and creation of biocompatible scaffolds are important issues for heart valve tissue engineering [Citation1].
The goal of any decellularization procedure is to remove all of the cellular components from the extracellular matrix (ECM) while preserving biological activity and mechanical integrity. Decellularization protocols may involve any combination of physical, chemical, or enzymatic methods and utilize a variety of detergents, enzymes, or solvents to lyse the native cells from the ECM. The treatments effectively reduce antigenicity while creating free volume spaces upon which the host's native cells are able to proliferate [Citation2–3]. However, increasing evidence that these decellularized porcine matrices remain immunogenic with the potential to trigger an intense cell-mediated immune response and calcification has been demonstrated [Citation4–5]. Moreover, the removal of cells will also alter the three-dimensional architecture of the ECM. Furthermore, during decellularization a significant amount of glycosaminoglycans (GAGs) are lost [Citation6].
GAGs are critical to the function of numerous soft connective tissues, including heart valves, providing material properties such as viscoelasticity and resistance to compression and tension [Citation7–8]. GAGs constitute a large fraction of the ECM of porcine aortic valve cusps, particularly within the central cuspal layer, the spongiosa. These molecules, because of the high concentration of negative charges and their inherent hydrophilicity, are capable of absorbing a large amount of water within the tissue matrix. The highly hydrated spongiosa layer serves as a cushion material to mediate deformations of the fibrous layers, allows shearing between the outer layers, and absorbs compressive forces during valve function [Citation9–10]. As a result, GAGs are important components of the ECM of native heart valve cusps, especially with regards to mechanical behavior [Citation11–13]. Other important biological characteristics of GAGs include the binding of growth factors, inhibition of proteases, and involvement in adhesion, migration, proliferation, and differentiation of cells [Citation14]. The loss of the GAG-rich spongiosa layer from porcine bioprosthetic aortic valves is thought to be instrumental in the failure of these devices [Citation15–17]. Lack of this buffering layer results in large shear stress and tissue buckling when subjected to bending [Citation18]. Since these GAGs are so important in heart valve development and function, the preservation of these molecules is indispensable in the development of a tissue engineered heart valve (TEHV) [Citation19].
In this study, we aim to evaluate the effect of four different decellularization methods on the extracellular matrix of porcine aortic valve leaflets, its mechanical strength, and biocompatibility. Immunogenicity and calcification potential of the matrices were evaluated in the rat model.
MATERIALS AND METHODS
Matrix Preparation
Porcine hearts were obtained under clean conditions from a local slaughterhouse, transferred to the laboratory in 0.9% NaCl (4°C), and immediately processed. Aortic valves were excised. They were immediately washed in 0.9% NaCl containing phenyl-methylsulfonylfluoride (PMSF: 1μM, Sigma, Bornem, Belgium) and antibiotics (streptomycin: 100U/ml; penicillin: 100U/ml mixture; Sigma), followed by an overnight decontamination at 4°C in Dulbecco's modified Eagle's medium (DMEM, Invitrogen, Belgium) supplemented with antibiotics (1.2mg amikacin; 3mg flucytosin; 1.2mg vancomycin; and 1.2mg metronidazol in 1ml aqua ad inject).
Decellularization
For decellularization of the aortic valves four different methods were applied: the trypsin enzymatic cell extraction, the osmotic procedure, the trypsin-osmotic method, and the non-ionic detergent-osmotic treatment. Control valves were native non-decellularized leaflets.
Trypsin Treatment. Following sterilization, the valves were placed in a solution of 0.05% trypsin and 0.02% EDTA (pH 8.0) (Invitrogen) at 37°C for 4 hours under continuous shaking. The solutions were supplemented with PMSF (1μM), penicillin/streptomycin solution (100U/ml, respectively), and 50μM butylated hydroxyanisole (Sigma). The valves were then washed for another 72 hours with PBS at 4°C under gentle agitation to remove residual substances.
Osmotic Treatment. Leaflets were subjected to a hypotonic solution (10mM Trizma, 5mM EDTA, pH 8.0) for 48 hours followed by a 48-hour treatment in a hypertonic solution (10mM Trizma HCl, 5mM EDTA, pH 8.0) at 4°C under gentle agitation. Solutions were supplemented with PMSF (1μM), penicillin/streptomycin solution (100U/ml, respectively), and 50μM butylated hydroxyanisole (Sigma). The valves were rinsed for 72 hours with PBS at 4°C under gentle shaking.
Trypsin-osmotic Treatment. Aortic valves were placed in a hypotonic solution (10mM Trizma, 5mM EDTA, pH 8.0) for 12 hours followed by a 12-hour treatment in a hypertonic solution (10mM Trizma HCl, 5mM EDTA, pH 8.0) at 4°C under gentle agitation. Solutions were supplemented with PMSF (1μM), penicillin/streptomycin solution (100U/ml, respectively), and 50μM butylated hydroxyanisole (Sigma). After thorough rinsing in PBS, tissues were placed in a 0.05% trypsin and 0.02% EDTA solution at 37°C for 4 hours under continuous shaking. Afterwards, the valves were washed for 72 hours with PBS at 4°C under agitation.
Detergent-osmotic Treatment. Tissues were subjected to a hypotonic Tris-buffer solution (pH 8.0) for 48 hours followed by a hypertonic 0.5% Triton X-100 solution for 48 hours at 4°C under gentle shaking. Solutions were supplemented with PMSF (1μM), penicillin/streptomycin solution (100U/ml, respectively), and 50μM butylated hydroxyanisole (Sigma). Valves were rinsed for 72 hours with PBS at 4°C under gentle agitation.
Mechanical Testing
A Loyd LF Plus universal material tester (Analis NV, Suarleé, Belgium) with a 1000 Newton (N) load cell in combination with the build in “Compress” test program and NexygenTM MT software were used for the analysis. A valve leaflet was placed over a 6.1-mm hole between two metal plates. Subsequently, a ball probe with a diameter of 4.45 mm approached the leaflet through the hole at a speed of 100 mm/min. After applying a preload of 0.2 N, the test started and the ball probe was further depressed into the leaflet until breakage. Experiments were performed in triplicate. Matrices used for tensile strength testing were not implanted. Control leaflets were native non-decellularized valves.
Animals
Nine 6-week old Wistar rats weighing approximately 250g each were obtained from Harlan Laboratories (Horst, The Netherlands). Animals were allowed to acclimatize to the Animal Facilities at our institution for 1 week before the investigations. All animal care complied with The Guide for the Care and Use of Laboratory Animals (1996) [Citation20]. All of our protocols involving animals were approved by the Ethical Commission for Animal Experiments of the University of Ghent (Project No. ECP 09/54).
Implantation and Explantation of Leaflets
Subcutaneous implantation in rats is the standard FDA accepted technique for evaluating bioprosthetic tissue valve materials. All animals were anesthetized using 1–2% isoflurane by inhalation. Following anaesthesia, four subcutaneous pouches were created in the abdomen of each animal. Leaflets (n = 4) were implanted in each animal as follows. In the right upper quadrant, a trypsin treated leaflet and in the right lower quadrant a trypsin-osmotic decellularized leaflet. In the corresponding upper and lower quadrants of the left side, the osmotic and detergent-osmotic treated leaflets were implanted, respectively. All leaflets were thoroughly rinsed in 0.9% NaCl before implantation. After recovery from anaesthesia, all animals were returned to the animal facilities and fed a standard rat diet. Animals were sacrificed after one week. After sacrifice, each leaflet was divided into two segments by cutting the free edge down towards the base. One segment was fixed in 4% formaldehyde for histological examination and another segment was fixed in glutaraldehyde for transmission electron microscopy.
Histology
Light Microscopy. Samples of decellularized leaflets were fixed in 4% phosphate buffered formaldehyde (Merck, Darmstadt, Germany) and embedded in paraffin. Five-micron-thick sections were cut and stained with Haematoxylin/Eosin (HE). A Russell-Movat Pentachrome stain kit (Mastertechs, Lodi, CA, USA) was used to demonstrate extracellular matrix components such as collagen, elastin, and glycosaminoglycans (GAGs). Samples of explanted valves were stained with Haematoxylin/Eosin. Von Kossa staining was used to evaluate tissue calcification.
Electron Microscopy. Samples of non-implanted valves were fixed in phosphate buffered solution of 4% formaldehyde (Merck, Darmstadt, Germany), supplemented with Alcian blue to precipitate and preserve the GAGs during the dehydratation. Specimens were subsequently post-fixed with 1% osmium tetroxide (OsO4) in phosphate buffer and embedded in epoxy resin. Ultra-thin 60 nm sections were cut and examined with a Jeol 1200 EX-II transmission electron microscope at 80 kV (Jeol, Zaventem, Belgium).
Immunohistochemistry. Explanted valves were deparaffinized. Endogenous peroxidase was quenched (0.06% hydrogen peroxide/methanol), nonspecific staining was blocked with normal horse serum, and sections were subsequently incubated with primary mAb for 1 hour at 37°C. The antibodies used were anti-CD3 (1F4, Cedarlanelabs, Burlington, Canada) specific for αβ T cell receptor-positive-bearing cells, and anti-CD8 (MRC OX-8, Cedarlanelabs, Burlington, Canada) specific for cell surface molecules present on CD8 + cytotoxic T lymphocytes. Sections were then washed in phosphate-buffered saline solution containing 1% bovine serum albumin (Sigma, Bornem, Belgium) three times before incubation with biotinylated secondary antibody and labelling with peroxidase avidin/biotin complex using 3.3'diaminobenzidine as the chromogen (Vector Laboratories, Inc, Burlingame, Calif).
Statistics
Descriptive statistics are expressed as mean ± standard deviation. One Way Analysis of Variance on Ranks (Kruskal-Wallis) was used for non-parametric analysis of mechanical testing data (SPSS 17.0, SPSS Inc, Chicago, IL, USA). If homogenicity was assumed by Kolmogorov-Smirnov Test an independent T-Test was used for parametric variables. A p value < 0.05 was considered significant.
RESULTS
Histology
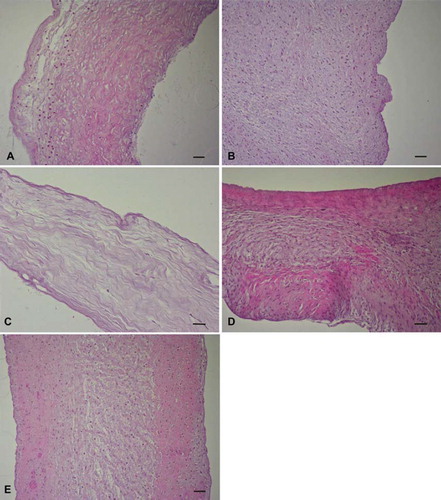
Light Microscopy. HE-stained sections of trypsin-treated leaflets revealed an incomplete cell removal from the matrix. Remnant cells were mainly found in the fibrosa and ventricularis. The spongiosa layer was almost completely acellular (). Both the osmotic () and detergent-osmotic () procedures did not remove any cells from the leaflets. Histology of trypsin-osmotic decellularized valve tissues showed that native porcine aortic valves were converted in an almost cell-free scaffold (). Only a few interstitial cells were present in the matrix.
Figure 1. HE stained (A) trypsin, (B) osmotic, (C) trypsin-osmotic, (D) detergent-osmotic decellularized, and (E) control leaflets. Cells were mostly detectable in the fibrosa and ventricularis of trypsin treated valves (A). Osmotic (B) and detergent-osmotic (D) treatment did not remove any cells from the valves when compared to controls. Trypsin-osmotic decellularization (C) resulted in an almost completely acellular matrix. Only a few interstitial cells are present. Scale bar 50μm.
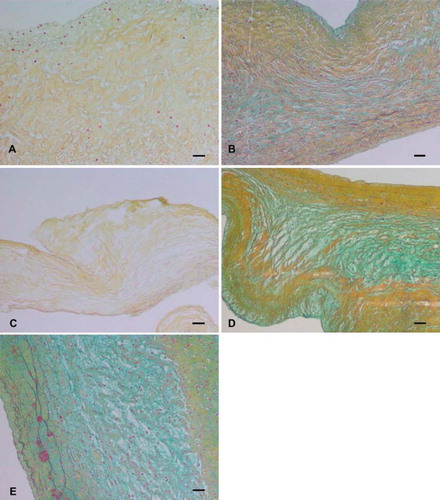
Russell-Movat pentachrome staining indicated that the three-layered structure of the leaflets was preserved in osmotic () and detergent-osmotic treated matrices () comparable to control leaflets (). These matrices showed preservation of ECM components and organized layered tissue formation within the fibrosa and ventricularis by detection of staining for collagen (yellow/greenish yellow) and several elastic fibers (black). The spongiosa demonstrated loose, amorphous ECM staining positive for sulphated and carboxylated acid GAGs and sialomucins (glycoproteins) (light blue/bluish green). Tryspin () and trypsin-osmotic () treated matrices showed a significant loss of collagen. No elastic fibers or GAGs were detectable. These treatments resulted in a destruction of the leaflet histo-architecture and matrix integrity. Shown sections are representative of all researched specimens.
Figure 2. Russel-Movat pentachrome staining of (A) trypsin, (B) osmotic, (C) trypsin-osmotic, (D) detergent-osmotic, and (E) control leaflets. The three-layered structure of the leaflets was preserved in all matrices except those of (A) trypsin and (C) trypsin-osmotic treatments. Trypsin-based treatments resulted in a significant loss of collagen. No elastic fibers or GAGs were detectable. Scale bar 50μm.
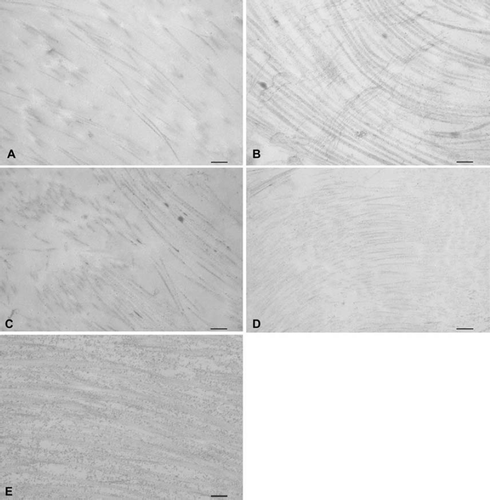
Electron Microscopy. Transmission electron microscopy showed that collagen structure was well preserved in all matrices except trypsin () and trypsin-osmotic treated valves (). No tightly packed and aligned collagen fibers could be observed. Collagen fibers have a lose association with each other and the extra-collagenous matrix was not electron dense.
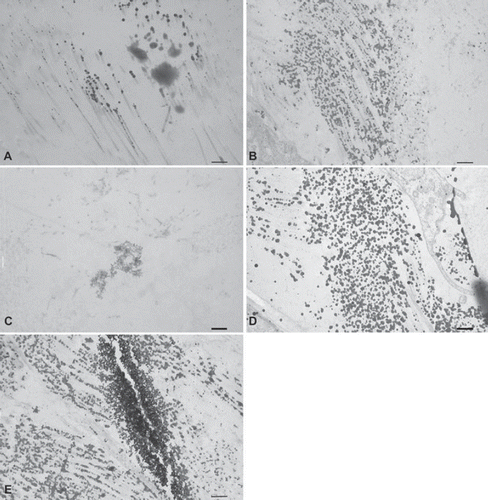
Figure 3. Transmission electron microscopic images of (A) trypsin, (B) osmotic, (C) trypsin-osmotic, (D) detergent-osmotic decellularized, and (E) control leaflets. In trypsin (A) and trypsin-osmotic (C) matrices, tightly packed and aligned collagen fibres are absent. Collagen fibres of detergent-osmotic matrices (D) show a decreased density. The collagen structure of the osmotic treated valves (B) is well preserved compared to controls (E). Scale bar 500nm.
Osmotic treated valves () show no collagen abnormalities as compared to controls (). Tightly packed collagen fibrils remained intact and maintained their normal diameter and banding pattern. Detergent-osmotic treated valves show smaller and less densely packed collagen fibers () when compared to controls ().
Trypsin treatment () resulted in a significant reduction of the amount of GAGs when compared to the osmotic (), detergent-osmotic (), and control matrices (). In the trypsin treated valves only a few electron dense precipitates of GAGs appeared clumped and did not seem to crosslink adjacent fibers (). Moreover, these GAGs appeared to be smaller when compared to the other treated matrices. In the osmotic treated leaflets () GAGs are closely associated with the collagen fibers comparable to control valves (). The precipitates in detergent-osmotic valves are preserved but appear to be clumped and less associated with collagen fibers since the collagenous matrix was organized more diffusely (). The trypsin-osmotic treated valves showed a complete loss of GAGs ().
Figure 4. Transmission electron microscopic images of (A) trypsin, (B) osmotic, (C) trypsin-osmotic, (D) detergent-osmotic decellularized, and (E) control leaflets. (A) shows some remnants of GAGs. In osmotic matrices (B) GAGs are well preserved and closely associated with collagen fibres compared to controls (E). Detergent-osmotic matrices (D) show GAG preservation, but less association with collagen fibres. A complete loss of GAGs is present in trypsin-osmotic leaflets (C). Scale bar 500nm.
Mechanical Testing
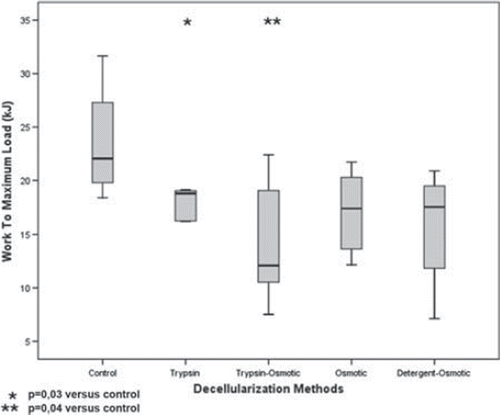
Work to maximum load (kJ) ± SD was measured for trypsin: 17,10 ± 2,61; osmotic:15,73 ± 3,91; trypsin-osmotic: 13,95 ± 5,63; detergent-osmotic: 16,61 ± 5.22 and control matrices: 23,54 ± 5. Mechanical strength of trypsin (p = 0,03) and trypsin-osmotic treated matrices (p = 0,04) was significantly lower compared to control matrices (). No significant difference in mechanical strength could be observed between the other decellularization methods (p > 0.05).
Explant Analysis
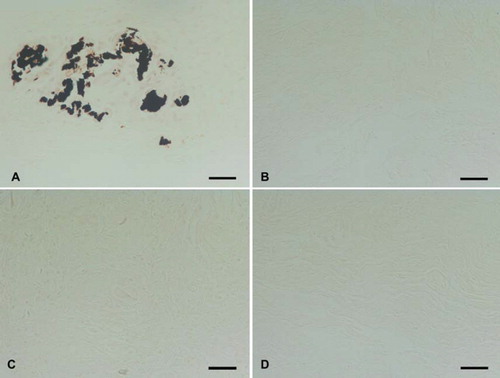
All explanted leaflets showed CD3 + T-cell inflammatory infiltration. Trypsin treated matrices show a strong infiltration of CD3 + cells () with only a few CD8 + cytotoxic T lymphocytes (). Inflammatory cells were observed inside the scaffolds. Matrices also showed fibrotic tissue encapsulation and neo-vascularisation accompanied with calcific deposits as confirmed by von Kossa staining (). All other treated matrices showed no signs of calcification ().
Figure 6. Immunohistochemical CD3+ T-cell staining seven days after implantation of (A) trypsin, (B) osmotic, (C) trypsin-osmotic, (D) detergent-osmotic treated matrices. Trypsin (A) and trypsin-osmotisc matrices (C) show a strong inflammatory infiltration. Osmotic leaflets (B) show a milder inflammatory response. Only a few CD3 + T-cells are present in detergent-osmotic matrices (D). Scale bar 100μm.
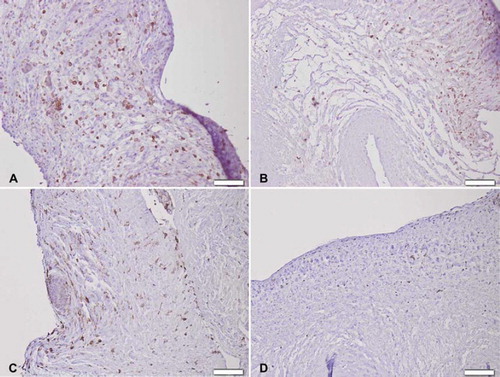
Figure 7. Immunohistochemical CD8 + T lymphocyte staining seven days after implantation of (A) trypsin, (B) osmotic, (C) trypsin-osmotic, and (D) detergent-osmotic treated matrices. Only trypsin (A) and detergent-osmotic matrices (D) show some CD8 + T-lymphocytic infiltration. Scale bar 100μm.
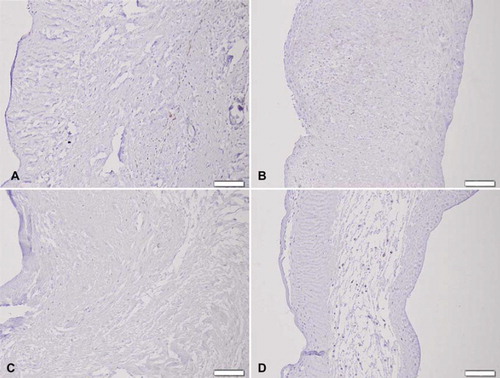
Figure 8. Von Kossa staining of (A) trypsin, (B) osmotic, (C) trypsin-osmotic, and (D) detergent-osmotic treated matrices seven days after implantation. Calcific deposits are only present in trypsin matrices (A), as indicated by black staining. Scale bar 100μm.
Osmotic decellularized matrices show a milder CD3 + inflammatory reaction () when compared to trypsin treated matrices. Inflammatory cells were mainly found at the edges of the scaffolds. No CD8 + cells could be detected (). In detergent-osmotic matrices only a few CD3 + and CD8 + cells could be identified (, ). Trypsin-osmotic matrices evoke a strong CD3 + cell infiltration inside the scaffolds (). CD8 + cytotoxic T lymphocytes are absent ().
DISCUSSION
Extracellular matrix proteins such as collagen, elastin, and GAGs are well-known critical elements for the structural integrity and biomechanical profile of heart valves [Citation21]. Moreover, a design close to the human model is the major motivation to use xenogeneic biological matrices for heart valve tissue engineering.
The present study focused on finding the most effective, ECM- and GAG-preserving method to produce non-immunogenic decellularized heart valve scaffolds. The goal of any decellularization protocol is to efficiently remove all antigenic cellular and nuclear materials while minimizing any adverse effect on the composition, biological activity, and mechanical integrity of the remaining ECM.
Using a 0.05% trypsin-0.02% EDTA method for 4 hours resulted in an almost completely acellular spongiosa. However, cell removal was clearly insufficient in the ventricularis and fibrosa. Most remnant cells that appeared round and shrunken had lost contact with the ECM. Subtraction of the cells from the leaflet was impossible, even with extended washings up to 72h. Prolonged treatment with trypsin for up to 24h further reduced the cell numbers, but did not result in a completely acellular leaflet. Moreover, our study indicates that already 4 hours of trypsin treatment resulted in substantial collagen damage and loss confirmed by TEM and Russel-Movat pentachrome staining. Remaining collagen fibers presented as a loose maze, which can be partly explained by a significant loss of GAGs known to crosslink all fibers. The near acellular spongiosa and washout of GAGs indicate a decellularization from the inside to the outside of the scaffold.
Trypsin treatment has previously been reported to be a successful method for decellularization of ovine [Citation22] and human [Citation23] heart valves. Despite the exposure to a greater concentration of trypsin (0.5% versus 0.05%) over a similar time-span, Grauss et al. found that a trypsin-based decellularization protocol was ineffective in removing the cellular material from the rat aortic heart valve [Citation24]. By contrast, Schenke-Layland and co-workers showed complete removal of cells from a porcine pulmonary valve [Citation25]. In our study, treatment of porcine aortic valves for a period of 4h or even 24h did not result in a sufficient removal of leaflet cells. Moreover, it has been demonstrated that treatment for up to 96h failed to produce completely acellular valves with multiple residual nuclei within the matrix [Citation26]. The contradictory results described in the literature could be caused by the type of trypsin used. Also, trypsin concentration, duration of the procedure, presence of protease inhibitors and species differences could all be of influence, too [Citation27]. In our study, PMSF was used to reduce the effect of protease activation. Therefore it is not very likely that observed differences in ECM damage were caused by the protease activation.
Osmotic and detergent-osmotic treatments did not remove cells from the matrices. Previously, we used a patented detergent-enzymatic of 1% Triton X-100 concentration, which is known to remove a significant amount of GAGs [Citation6, Citation28–29]. Also, Grauss and co-workers demonstrated that Triton X-100 can lead to a complete loss of GAGs in the valve tissue [Citation24]. Therefore, in this study we lowered detergent concentration to 0.5% in order to diminish GAG loss. As confirmed by Russell-Movat pentachrome staining and TEM, the lower Triton X-100 concentration better preserves GAGs in the leaflets.
Non-ionic detergents such as Triton X-100 are known because of their mild effects upon tissue structures. They disrupt lipid-lipid and lipid-protein interactions, but leave protein-protein interactions intact. Therefore tissue proteins following Triton X-100 treatment should appear in their functional confirmation [Citation30]. However, decellularization of tissues with Triton X-100 has shown mixed results. Our results are consistent with the study of Kim et al., which also failed to obtain effective decellularized valves using Triton X-100 [Citation31]. Other studies have reported that even exposure for up to 4 days was not effective at completely removing cellular material [Citation32–33]. Moreover, it was found that Triton X-100 treatment can severely alter the tensile strength of collagen fibers in tendons [Citation34]. In our study, 0.5% Triton X-100 treatment resulted in a decrease of collagen density with widening of the inter-fibrillar spaces consistent with earlier findings [Citation35]. However, our results indicate that these minor changes in the quantity of the collagen fiber network do not result in a significant loss of valve's mechanical strength.
Trypsin-osmotic decellularization removed almost all cells from the matrices. Only a few interstitial cells were still present. The collagen ultrastructure of the leaflets was severely damaged. Moreover, trypsin-osmotic treatment resulted in a complete loss of elastin and GAGs. Cell extraction by trypsin alone resulted in a significant loss and less compact appearance of collagen bundles. ECM components such as elastin and GAGs were also completely lost. It is clear that trypsin treatment significantly decreases mechanical strength of the matrices. Both trypsin-osmotic (p = 0,04) and trypsin-treated matrices (p = 0,03) show inferior mechanical strength compared to non-decellularized control leaflets. The effects on the collagen and elastin ultrastructure result in a decrease of mechanical strength of these matrices, as demonstrated in this study. Moreover, the loss of GAGs, which serve as a cushioning layer, makes these valves less resistant to deformation [Citation35]. However, no significant difference in mechanical strength could be observed among the four treatments (p > 0.05). Although collagen ultrastructure and GAGs appeared more preserved in osmotic and detergent-osmotic leaflets, these treatments obviously also resulted in a weakening of the valve's mechanical strength. This is probably due to an alteration of the three-dimensional architecture of the ECM, which leads to a decrease in tensile strength in all treated matrices.
The osmotic shock with hypotonic and hypertonic solutions implemented in the trypsin-osmotic procedure was used to disrupt the cell membrane, release cell contents, and facilitate subsequent rinsing and removal of the cell contents from the ECM [Citation36–37]. However, this treatment alone does not remove all cellular remnants from the tissue [Citation36]. Thus an additional tryspin-enzymatic method was used to disrupt cell membranes and the bonds responsible for intercellular and extracellular connections. A hypothesis for the near acellular matrices is that, due to the cell lysis induced by osmotic shock, the primary target of trypsin becomes the removal of all cellular debris by cleaving peptide bonds on the carbon side of arginine and lysine instead of attacking proline, a major amino acid component of collagen [Citation38]. The use of trypsin alone clearly cleaves less cellular debris; however, it also affects proline residues on the collagen fibers. Moreover, we implemented a long washing step of 72h to wash out residual cell debris and remove residual agents to avoid an adverse host tissue response. Most studies report washing steps of maximum 24 to 48 hours. It has been demonstrated that a large amount of cell debris remains in non-washed valves [Citation26]. Furthermore, all the steps were performed under mechanical agitation to increase their effectiveness. The optimal speed, volume of reagent, and length of mechanical agitation is dependent on the composition, volume, and density of the tissue [Citation39].
In-vivo evaluation in the rat model showed a strong CD3 + T-cell inflammatory infiltration accompanied with calcific deposits after one week in the trypsin-treated matrices. No other matrices did show any signs of calcification. The collagenous matrix of trypsin treated matrices is probably more damaged compared to trypsin-osmotic leaflets, since mineralization of biological valves usually begins at the sites of disrupted collagen fibers. Clearly, the loss of GAGs in these scaffolds is not responsible for calcification. It has been previously speculated that the presence of negatively charged GAG molecules within the ECM of cuspal tissue may reduce calcification by chelating calcium ions, thereby preventing hydroxyapatite nucleation [Citation40–41]. However, the GAG-depleted trypsin-osmotic matrices show no calcific deposits. Trypsin-osmotic treated valves also evoked a strong CD3 + inflammatory reaction. This indicates that the near cell removal is not sufficient to minimize an in-vivo immune reaction. One of the first questions regarding the immunogenicity of xenogeneic valves is which of their components are immunogenic. Our results indicate that both trypsin and trypsin-osmotic near acellular matrices evoke a more intense T-cell mediated inflammatory reaction compared to osmotic and detergent-osmotic matrices with no cells removed. This clearly demonstrates that resident antigenic cells are not solely responsible for the immune host response. The immunogenicity of porcine heart valve matrices is clearly supported by the ECM in addition to the cells. Morwood and colleagues have demonstrated that ECM proteins are known to play an important role in regulating immune reaction [Citation42]. Some of the minor ECM proteins have been grouped together as “matricellular proteins,” whose function is achieved by binding both matrix and cell surface receptors. Some of these proteins have functions that are primarily functional rather than structural. Fragments of specific ECM proteins have strong pro-inflammatory functions and augment the immune response both by activating the innate immune response and by acting as regulators of inflammatory cell apoptosis [Citation43]. The ECM disruption due to the tryspin-based decellularization process probably allows for adequate exposure of these matricellular proteins to trigger a T-cell mediated immune response through the coordinated interaction of adhesion molecules with their receptors, both present on inflammatory cells and within the ECM. The evoked in-vivo immune response was mainly CD3 + T-cell mediated since only a few cytotoxic T-cells, mainly implicated in transplant rejection, could be identified.
Limitations of the Study
A limitation of the study is that the porcine matrices were implanted subcutaneously in the abdominal wall of rats and thus were not exposed to mechanical forces, as in a circulatory system. Immune reaction, inflammation, and calcification will be even more prominent in a dynamic environment. Our next step will be the implantation of these matrices in the pulmonary position in the sheep model for six weeks. Also, the effect on cell proliferation, migration, and invasion needs to be investigated.
CONCLUSION
Of all treatments only trypsin-osmotic effected an almost complete cell removal of porcine heart valve matrices. However, it resulted in a major disruption and loss of ECM components.
Despite this cell removal, matrices still evoke a strong inflammatory and T-cell mediated immune reaction. One possible method for attenuating immune reaction could be the use of a bio-friendly crosslinker such as genipin. Our previous experiments demonstrated that genipin fixation can mitigate all traces of inflammation by inhibiting in-vivo immune cell penetration. Moreover, genipin can increase mechanical strength and therefore extend the durability of these matrices [Citation44].
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Knight, R.L., Wilcox, H.E., Korossis, S.A., Fisher, J., Ingham, E. (2008). The use of acellular matrices for tissue engineering of cardiac valves. Proc Inst Mech Eng 222: 129–143.
- Rieder, E., Seebacher, G., Kasimir, M.-T., . (2005). Tissue engineering of heart valves: Decellularized porcine and human valve scaffolds differ importantly in residual potential to attract monocytic cells. Circulation 111(21): 2792–2797.
- Gilbert, T.W., Sellaro, T.L., Badylak, S.F. (2006). Decellularization of tissues and organs. Biomaterials 27(19): 3675–83.
- Van Nooten, G., Somers, P., Cornelissen, M., Bouchez, S., Gasthuys, F., Cox, E., Sparks, L., Narine, K. (2006). Acellular porcine and kangaroo aortic valve scaffolds show more intense immune-mediated calcification than cross-linked Toronto SPV® valves in the sheep model. Interact Cardiovasc Thorac Surg 5(5): 544–549.
- Bastian, F., Stelzmüller, M.E., Kratochwill, K., Kasimir, M.T., Simon, P., Weigel, G. (2008). IgG deposition and activation of the classical complement pathway involvement in the activation of human granulocytes by decellularized porcine heart valve tissue. Biomaterials 29(12): 1824–1832.
- Narine, K., Claeys, E., Cornelissen, M., De Somer, F., Beele, H., Vanlangenhove, L., De Smet, S., Van Nooten, G. (2006). Readily available porcine aortic matrices for use in tissue valve engineering. Is cryopreservation an option? Cryobiology 53(2): 169–181.
- Kinsella, M.G., Bressler, S.L., Wight, T.N. (2004). The regulated synthesis of versican, decorin, and biglycan: Extracellular matrix proteoglycans that influence cellular phenotype. Crit Rev Eukaryot Gene Expr 14(3): 203–234.
- Rothenburger, M., Volker, W., Vischer, P., Glasmacher, B., Scheld, H.H., Dewick, M. (2002). Ultrastructure of proteoglycans in tissue-engineered cardiovascular structures. Tissue Eng 8(6): 1049–1056.
- Talman, E.A., Boughner, D.R. (1995). Glutaraldehyde fixation alters the internal shear properties of porcine aortic heart valve tissue. Ann Thorac Surg 60: 369–373.
- Talman, E.A., Boughner, D.R. (1996). Internal shear properties of fresh porcine aortic heart valve cusps: Implications for normal valve function. J Heart Valve Dis 5(2): 152–159.
- Schoen, F.J., Levy, R.J. (1999). Tissue heart valves: Current challenges and future research perspectives. J Biomed Mater Res 47(4): 439–465.
- Human, P., Zilla, P. (2001). Characterization of the immune response to valve bioprostheses and its role in primary tissue failure. Ann Thorec Surg 71(5): 385–388.
- Simionescu, D.T., Lovekamp, J.J., Vyavahare, N.R. (2003). Degeneration of bioprosthetic heart valve cusp and wall tissues is initiated during tissue preparation: An ultrastructural study. J Heart Valve Dis 12(2): 226–234.
- Jackson, R.L., Busch, S.J., Cardin, A.D. (1991). Glycosaminoglycans: Molecular properties, protein interactions, and role in physiological processes. Physiol Rev 71(2): 481–539.
- Sacks, M.S. (2001). The biomechanical effects of fatigue on the porcine bioprosthetic heart valve. J Long Term Eff Med Implants 11(3–4): 231–247.
- Vesely, I., Mako, W.J. (1998). Comparison of the compressive buckling of porcine aortic valve cusps and bovine pericardium. J Heart Valve Dis 7(1): 34–39.
- Smith, D.B., Sacks, M.S., Pattany, P.M., Schroeder, R. (1999). Fatigue-induced changes in bbioprosthetic heart valves three-dimensional geometry and the relation to tissue damage. J Heart Valve Dis 8(1): 25–33.
- Vesely, I., Boughner, D., Song, T. (1988). Tissue buckling as a mechanism of bioprosthetic valve failure. Ann Thorac Surg 46(3): 302–308.
- Aikawa, E., Whittaker, P., Farber, M., Mendelson, K., Padera, R.F., Aikawa, M. . (2006). Human semilunar cardiac valve remodelling by activated cells from fetus to adult: Implications for postnatal adaptation, pathology, and tissue engineering. Circulation 113(10): 1344–1352.
- Guide for the Care and Use of Laboratory Animals, Publ 85: 23, modified 85, National Institutes of Health, Bethesda, MD.
- Bader, A., Schilling, T., Teebken, O.E., . (1998). Tissue engineering of heart valves: Human endothelial cell seeding on detergent acellularized porcine valves. Eur J Cardiothorac Surg 14: 279–284.
- Steinhoff, G., Stock, U., Karim, N., Mertsching, H., Timke, A., Meliss, R.R., Pethig, K., Haverich, A., Bader, A. (2000). Tissue engineering of pulmonary heart valves on allogenic acellular matrix conduits: In vivo restoration of valve tissue. Circulation 102:III50–III55.
- Cebotari, S., Mertsching, H., Kallenbach, K., Kostin, S., Repin, O., Batrinac, A., Kleczka, C., Ciubotaru, A., Haverich, A. (2002). Construction of autologous human heart valves based in an acellular allograft matrix. Circulation 106(12): I63–I68.
- Grauss, R.W., Hazekamp, M.G., Oppenhuizen, F., van Munsteren, C.J., Gittenberger-de Groot, A.C., DeRuiter, M.C. (2005). Histological changes of decellularized porcine aortic valves: Matrix changes due to different decellularisation methods. Eur J Cardio-Thorac Surg 27: 566–571.
- Schenke-Layland, K., Vasilevski, O., König, K., Riemann, I., Halbhuber, K.J., Wahlers, T.H., Stock, U.A. (2003). Impact of decellularization of xenogeneic tissue on extracellular matrix integrity for tissue engineering of heart valves. Journal of Structural Biology 143: 201–208.
- Kasimir, M.T., Rieder, E., Seebacher, G., Silberhumer, G., Wolner, E., Weigel, G., Simon, P. (2003). Comparison of different decellularization procedures of porcine heart valves. Int J Artif Organs 26: 421–427.
- Mol, A., Bouten, C.V., Baaijens, F.P., Zund, G., Turina, M.J., Hoerstrup, S.P. (2004). Review article: tissue engineering of semilunar heart valves: Current status and future developments. J Heart Valve Dis 13(2): 272–280.
- Wilson, G.J., Courtman, D.W., Klement, Peter, Lee, J.M., Yeger, H. (1995). Acellular matrix: A biomaterials approach for coronary artery bypass and heart valve replacement. Ann Thorac Surg 60: 353–358.
- Zeltinger, J., Landeen, L.K., Alexander, H.G., Kidd, I.G., Sibanda, B. (2001). Development and characterisation of tissue-engineered aortic valves. Tissue Eng. 7(1): 9–22.
- Seddon, A.M., Curnow, P., Booth, P.J. (2004). Membrane proteins, lipids, and detergents: Not just a soap opera. Biochim Biophys Acta 1666: 105–117.
- Kim, W.G., Park, J.K., Lee, W.Y. (2002). Tissue-engineered heart valve leaflets: An effective method of obtaining acellularized valve xenografts. In J Artif Organs 25: 791–797.
- Dahl, S.L., Koh, J., Prabhakar, V., Niklason, L.E. (2003). Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant 12: 659–666.
- Cartmell, J.S., Dunn, M.G. (2000). Effect of chemical treatments on tendon cellularity and mechanical properties. J Biomed Mater Res 49: 134–140.
- Bader, A., Schilling, T., Teebken, O.E., Brandes, G., Herden, T., Steinhoff, G., Haverich, A. (1998). Tissue engineering of heart valves-human endothelial cell seeding of detergent acellularized porcine valves. Eur J Cardiothorac Surg 14: 279–284.
- Schoen, F.J., Levy, R.J. (1999). Tissue heart valves: Current challenges and future research perspectives. J Biomed Mater Res 47: 439–465.
- Dahl, S.L., Koh, J., Prabhakar, V., Niklason, L.E. (2003). Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant 12: 659–666.
- Goissis, G., Suzigan, S., Parreira, D.R., Maniglia, J.V., Braile, D.M., Raymundo, S. (2000). Preparation and characterization of collagen-elastin matrices from blood vessels intended as small diameter vascular grafts. Artif Organs 24: 217–223.
- Voet, D.,Voet, J.G., Pratt, C.W. (2002). Fundamentals of Biochemistry, Wiley, New York.
- Gilbert, T.W., Sellaro, T.L., Badylak, S.F. (2006). Decellularization of tissues and organs. Biomaterials 27: 3675–3683.
- Lovekanmp, J., Vyavahare, N. (2001). Periodate-mediated glycosaminoglycan stabilization in bioprosthetic heart valves. J Biomed Mater Res 56(4): 478–486.
- Hunter, G.K. (1987). An ion-exchange mechanism of cartilage calcification. Connect Tissue Res 16(2): 111–120.
- Morwood, S.R., Nicholson, L.B. (2006). Modulation of the immune response by extracellular matrix proteins. Arch Immunol Ther Exp 54: 1–8.
- Jiang, D., Liang, J., Fan, J., . (2005). Regulation of lung injury and repair by toll-like receptors and hyaluronan. Nat Med 11: 1173–1179.
- Somers, P., De Somer, F., Cornelissen, M., Bouchez, S., Gasthuys, F., Narine, K., Cox, E., Van Nooten, G. (2008). Genipin blues: An alternative non-toxic crosslinker for heart valves? J Heart Valve Dis 17(6): 682–688.