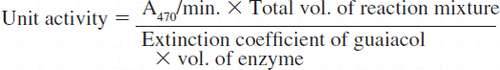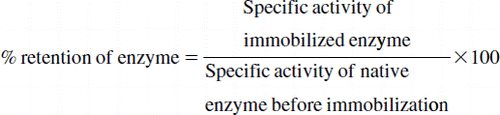Abstract
Abstract: A method is described for construction of an amperometric polyphenol biosensor employing nitrocellulose membrane-bound laccase purified from cell-free extract of Ganoderma lucidum onto a Pt electrode. The biosensor showed optimum response within 10s, at 0.4 V in 0.1M acetate buffer, pH 6.0, and 35°C. Detection limit of the biosensor was 3.0 × 10−8M. Analytical recovery of added guaiacol was 97.00%. Within batch and between batch coefficients of variation were <0.97% and <1.26%, respectively. The sensor measured total phenolic content in fruit juices and alcoholic beverages. The enzyme electrode was used 100 times over 4 months, when stored at 4°C.
INTRODUCTION
Polyphenols, one of the most important classes of natural antioxidants, widely occurring in fruits, vegetables, and medicinal plants, have been found to have a protective role against many chronic human diseases associated with oxidative stress, such as cancer or cardiovascular diseases [Citation1, Citation2]. Polyphenols have also been used as markers in taxonomic studies and food quality control [Citation3]. Methods reported for determination of phenolic compounds include spectrophotometric, enzyme assay, gas chromatography, capillary electrophoresis, high pressure liquid chromatography (HPLC), and gas chromatography-mass spectrometry [Citation4–8]. However, some of these techniques (e.g. spectrophotometry) are not sensitive and specific, while others (e.g. gas chromatography, liquid chromatography, and capillary electrophoresis) are cumbersome and require time-consuming sample pre-treatment, expensive equipment, and skilled persons to operate them [Citation9]. Therefore, there is an interest in developing a simple, sensitive, rapid, cost-effective and portable system such as a biosensor for determination of phenolic compounds in important areas such as clinical, biomedical, environmental, industrial, and pharmaceutical analysis. A number of biosensors for detection of phenolic compounds have been reported based on immobilization of laccase onto various supports such as sol gel film [Citation10], chitosan film [Citation11], graphite electrode [Citation12], polyethersulfone membrane [Citation13], nafion sol-gel silicate [Citation14], cellulose-based Granocel carriers [Citation15], and woven nylon supports [Citation16] through adsorption. All these biosensors suffer from leaking of enzyme and some drawbacks such as limited stability, reusability, and a complex method of immobilization, which restrict their use in development of polyphenol biosensors.
Laccases, multi-copper containing enzymes, are widely distributed in fungi, higher plants, and some bacteria [Citation17–19]. Laccase is a type of copper-containing polyphenol oxidase (p-diphenol oxidase, EC 1.10.3.2) that oxidizes polyphenols, but it does not oxidize tyrosine (as do the tyrosinases). Generally, laccases have four copper ions. One of the copper ions is the site for the oxidation of the phenolic compounds and the other three are related to the reduction of oxygen [Citation20, Citation21]. Polyphenol oxidase catalyzes the oxidation of phenols to o-quinones, which are highly reactive compounds. O-quinones thus formed undergo spontaneous polymerization to produce high-molecular-weight compounds or brown pigments (melanins). These melanins may in turn react with amino acids and proteins, leading to enhancement of the brown color produced (enzymatic browning) [Citation22].
In the case of a reusable enzyme-based biosensor, an enzyme is commonly required to be immobilized on, or in close proximity to, the surface of a transducer. As a consequence, immobilization strategies for enzymes are of paramount importance to preserve their biological activity [Citation23]. Over the years, many metods have been used to immobilize enzymes for use in biosensing.
Chitosan, the N-deacetylated derivative of chitin, is a natural polymer product consisting of a repeating hexosamide residue with one –NH2 and two -OH groups. It has excellent film-forming ability, biocompatibility, good adhesion, and high mechanical strength, which have led to growing interest in using it to immobilize biomolecules (especially enzymes) in recent years. It is also a stable electroactive material that allows the possible mass production of sensors [Citation24–30].
We report herein a simple procedure of covalent immobilization of laccase (purified from Ganoderma sp.) onto chitosan-decorated nitrocellulose membrane (cellulose nitrate, flash paper) and its application in construction of a polyphenol biosensor, as it is highly porous (pore size 200–450 nm), has high surface area, excellent uniformity and protein binding, and provides better anchoring feasibility due to diffusion of glutaraldehyde and enzyme. Further high affinity for enzyme, low cost, easy activation, and high mechanical strength are some additional advantages of this membrane.
EXPERIMENTAL
Materials
Sephadex G-100 and DEAE-Sephacel from Sigma Aldrich, and guaiacol and chitosan from SRL, Mumbai, were used. Nitrocellulose (NC) membrane was from Amersham Pvt. Ltd., Chennai. All other chemicals used were of AR grade. Fruit juices and alcoholic beverages of various commercial brands were purchased from a local market. Double distilled water (DW) was used throughout the experiments.
Purification of Laccase
The cell-free extract of Ganoderma sp. Rckk02 grown in malt extract broth (MEB) was used as a crude laccase [Citation31, Citation32]. The crude laccase was purified at 4oC using 0-80% ammonium sulphate precipitation, gel filtration on Sephadex G-100, and ion-exchange chromatography on DEAE-Sephacel using a linear gradient of KCl (0.1 M to 0.6 M). The fractions showing high specific activity were pooled and treated as purified enzyme. Finally, the enzyme was purified by 84.12-fold with 76.44 % yield. The purified enzyme had a specific activity of 531.96. Unit activity of enzyme was calculated as
Enzyme Assay
Assay of laccase was based on the oxidative polymerization of guaiacol [Citation31]. Ten micromolars of guaiacol in 100 mM acetate buffer (pH 5.0) were used as substrate. Change in absorbance of 0.01 min−1 ml−1 at 470 nm was measured.
Protein content in various enzyme preparations was determined by the Lowry method using bovine serum albumin as a standard protein.
Activation of NC Membrane
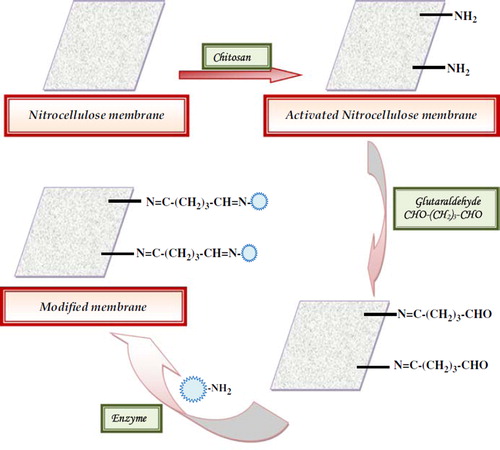
A rectangular piece of NC membrane (1 × 1.5 cm2) was first washed with DW to remove any debris and then air-dried for 30 min. To activate the membrane surface it was then treated with 5% chitosan solution (in 2% acetic acid) for 24 h at room temperature, which introduced a large number of free -NH2 groups on its surface. It was washed with 10% methanol and air-dried for 30 min.
Immobilization of Laccase on NC Membrane
The NC membrane was activated by immersing it into 2.5% glutaraldehyde in 0.02 M phosphate buffer pH 7.0 and then kept at room temperature for 2 h. This activated membrane was then washed thoroughly by 0.02 M phosphate buffer pH 7.0. Next, enzyme solution [laccase (97 μg protein containing 51.6 units, 1.0 ml)] was spread over the activated membrane and kept overnight at 4oC for covalent immobilization of the enzyme. The enzyme-modified membrane was washed with phosphate buffer (50 mM, pH 7.0) followed by DW to remove the unbound enzyme. The unbound enzyme in the washing discard was tested for activity and protein.
The efficiency of immobilization, i.e. % retention of enzyme activity after immobilization, is generally calculated as follows:
Construction of Amperometric Polyphenol Biosensor and Response Measurement
The working electrode was constructed by mounting the NC membrane containing immobilized laccase onto a Pt electrode using a parafilm. The working electrode, an Ag/AgCl as the reference electrode and a Cu wire as auxiliary electrode, were connected through a three-terminal electrometer/high resistance meter (Make: Keithley Japan Model 6517A/E). To test the activity of the biosensor, all three electrodes were dipped into a 4ml reaction mixture [1.0 ml guaiacol and 3.0 ml of 0.1 M acetate buffer pH 5.0]. The electrodes were polarized at different potential (in 0.1–0.6 V) and the current was measured through the electrometer. The maximum current was generated at 0.4 V, hence in subsequent amperometric experiments the current was read at 0.4V (mA). The current was also measured at varying concentrations of guaiacol in acetate buffer (0.1 M, pH 5.0). The principle of the working of this biosensor is that guaiacol was oxidized into its corresponding o-quinone by the NC-membrane-bound laccase and then regenerated through electrochemical reduction of o-quinone, thus forming a bioelectrocatalytic amplification cycle and thereby generating electrons, which are passed to the Pt electrode from the solution through pores of the NC membrane.
Study of Analytical and Kinetic Properties of Immobilized Laccase
Various kinetic properties of NC-membrane-bound enzyme such as optimum pH, incubation temperature, response time, and effect of substrate (guaiacol) concentration were studied amperometrically to determine the optimum working conditions of the biosensor.
Amperometric Determination of Total Phenolic Content in Fruit Juices and Alcoholic Beverages
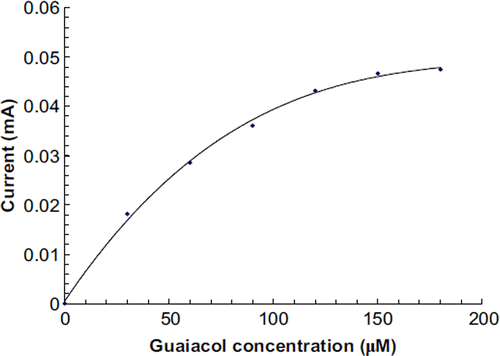
The total phenolic content in fruit juices and alcoholic beverages was determined by the biosensor under optimal working conditions. The procedure followed was the same as that described for response measurement of the biosensor, except that guaiacol was replaced by the fruit juice/alcoholic beverage. The current (mA) was recorded and the amount of total phenolic content was interpolated from a standard curve between guaiacol concentrations and current (mA) prepared under optimal working conditions ().
RESULTS AND DISCUSSION
Immobilization of Laccase onto “NC” Membrane
Laccase purified from Ganoderma Rckk02 sp. was immobilized covalently onto the “NC” membrane with 98% retention of initial activity of free enzyme and a conjugation yield of 0.063 mg/cm2. One –CHO group of glutaraldehyde was linked to the –NH2 group of enzyme and another –CHO group was bound to the –NH2 group of chitosan-activated NC membrane, providing a very strong and stable complex ().
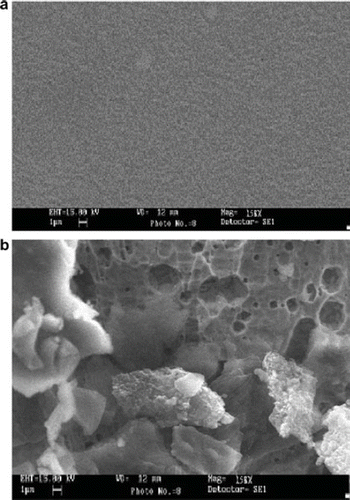
Scanning Electron Microscopic (SEM) Study of NC Membrane
The surface of the “NC” membrane without and with enzyme was observed under a scanning electron microscope (SEM) at the “Sophisticated Analytical Instrumentation Facility” of AIIMS, New Delhi, to confirm the immobilization. High-resolution scanning electron microphotographs of the NC membrane with the immobilized enzyme had protein-clustered structures that were not observed in the membrane without the immobilized enzyme (), which might be due to both the chemical coupling as well as the adsorption of the enzyme. A change in surface morphology of the membrane after the immobilization process is the evidence for immobilization of the enzyme.
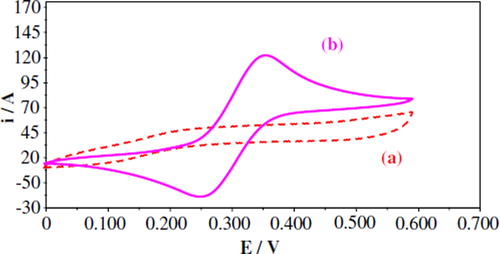
Cyclic Voltammetery Study
The modified membrane electrode without and with the immobilized enzyme was characterized by the cyclic voltammetric technique. shows a typical cyclic voltammogram obtained with the modified membrane biosensor in 0.1 M KCl (a) without and (b) with addition of 0.1 ml guaiacol solution (10 μM). An increase in the oxidation peak current at +0.35 V vs. Ag/AgCl was observed at +0.0 V to +0.6V with a scan rate of 50 mV s−1 when substrate was added to the solution.
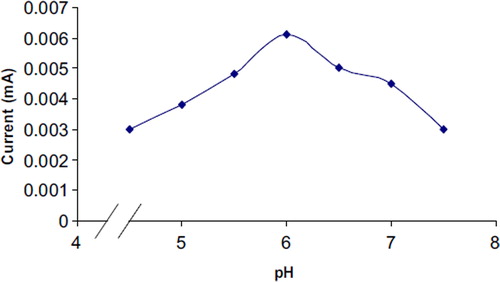
Optimum pH
The effect of pH of reaction buffer on the biosensor response was studied in the range of pH 4.5 to 6.5 using 10 mM acetate buffer and pH 7.0 to 8.0 using 10mM sodium phosphate buffer. The biosensor response increased with the increase in pH up to 6.0 and thereafter declined (). The optimum pH of the immobilized enzyme (pH 6.0) was higher than that of that immobilized on other supports, e.g. chitosan membrane modified with tripolyphosphate (pH 4.0) [Citation33], MWCNT chitosan composite (pH 4.5) [Citation34], and magnetic chitosan microspheres (pH 3.0) [Citation35], but lower than that immobilized on carbon-paste-modified graphite electrode (pH 7.0) [Citation36] and comparable to that of the platinum nanoparticles (pH 6.5) [Citation37] and silane-modified platinum surface (pH 6.0) [Citation38].
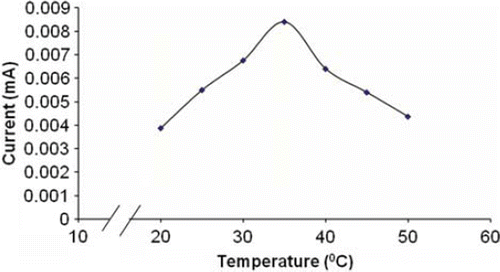
Optimum Incubation Temperature
The optimum temperature for the biosensor was 35°C (), which is lower than that immobilized onto magnetic chitosan microspheres (55°C) [Citation35] and chitosan (60°C) [Citation39], but higher than that immobilized on the carbon-paste-modified graphite electrode [Citation36] and laccase onto nanoparticles (25°C) [Citation40].
Effect of Substrate Concentration on Biosensor
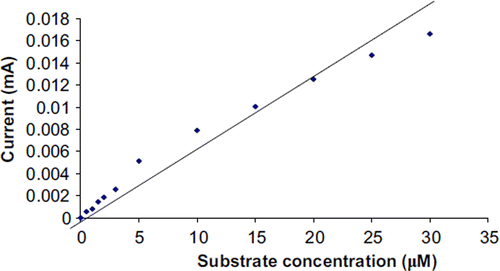
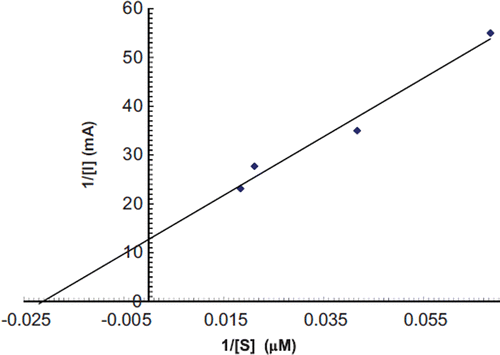
To study the effect of substrate concentration on biosensor response, the concentration of guaiacol was varied from 30 μM to 180 μM. A hyperbolic relationship was found between this guaiacol concentration range versus the current (in mA) (). Km for guaiacol, as calculated from the Lineweaver Burke plot, was 47.39 μM (), which is lower than that of magnetic chitosan microspheres (171.1 μM) [Citation35] and carbon-paste-modified graphite electrode (3.87 mM) [Citation36], but higher than that of the carbon nanotubes–chitosan composite (9.43 μM) [Citation41]. Imax was found to be 0.077 μmole/ml/min, which is higher than that of earlier reported results [Citation35]. The following criteria were studied to evaluate the performance of this biosensor.
Evaluation of the Performance of Biosensor
Linearity. There was a linear relationship between current (mA) and guaiacol concentration ranging from 5 × 10−7M to 3 × 10−5M (), which is better than earlier biosensors based on silane-modified platinum surface (0.6 × 10−6−15 × 10−6M) [Citation38], polyethersulphone membrane (1 × 10−5−8 × 10−5M) [Citation13], and carbon-paste-modified graphite electrode (1.97 × 10−4−3.24 × 10−3M) [Citation36], but comparable to those based on chitosan membrane modified with tripolyphosphate (5.99 × 10−7−3.92 × 10−6M) [Citation33] and carbon nanotubes-chitosan composite [3 × 10−7−1.2 × 10−6M] [Citation42].
Detection Limit. The detection limit of the biosensor was observed to be 3 × 10−8M, which is lower and better than that immobilized onto graphite electrode [2 × 10−6M] [Citation12], polyethersulphone membrane (1 × 10−5M) [Citation13], carbon-paste-modified graphite electrode (1.97 × 10−4M) [Citation36], carbon nanotubes-chitosan composite (6.6 × 10−7M) [Citation42], Cu-OMC/chitosan matrix (6.7 × 10−7M) [Citation43], and chitosan film (3.3 × 10−4 M) [Citation44], but comparable to those based on chitosan membrane modified with tripolyphosphate (6.23 × 10−8M) [Citation33].
Recovery. The percent recovery of added guaiacol in the grape juice sample (5.0 μM and 10.0 μM) by the present sensor was 94.00 and 97.00%, respectively (), indicating the good efficiency of the biosensor.
Table 1. Analytical recovery of added guaiacol in the grape juice, as measured by polyphenol biosensor based on nitrocellulose membrane-bound laccase.
Precision. To check the reproducibility and reliability of the biosensor, the total phenolic content in the five grape juice samples was estimated six times on a single day (within batch) and six times again in these samples after storage at −20°C for one week (between batch). The results showed that determinations were consistent and within and between CVs were <0.97 % and <1.26 %, respectively (). These results are comparatively better than the earlier reports [Citation45].
Table 2. Within and between assay coefficients of variation (CV) for determination of total phenolic content in the grape juice sample as measured by polyphenol biosensor based on nitrocellulose membrane-bound laccase.
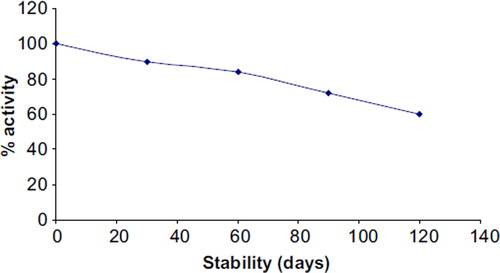
Reusability and Storage. To reuse the enzyme electrode, it was dipped in DW 3–4 times followed by reaction buffer before its use in the next assay. To store the immobilized enzyme, the NC membrane containing laccase was removed from the electrode and stored at 4oC in a Petri dish. The enzyme electrode lost 40% of its initial activity after its 100 uses during the span of 4 months when stored at 4oC (), which is much better than earlier biosensors [Citation38, Citation45, Citation46].
The properties of the biosensors tested here have been compared with other reported polyphenol biosensors ().
Table 3. A comparison of various analytical and kinetic properties of polyphenol biosensors based on laccase immobilized on different supports.
Determination of Total Phenolic Content in Fruit Juices and Alcoholic Beverages
Total phenolic content as measured by the present sensor ranged from 0.81–1.92 μM in fruit juices and 1.9–3.0 μM in alcoholic beverages ().
Table 4. Total phenolic content in different brands of fruit juices and alcoholic beverages as measured by polyphenol biosensor based on nitrocellulose membrane-bound laccase.
CONCLUSION
A polyphenol biosensor employing NC membrane-bound fungal laccase exhibited improved performance of biosensor in terms of linear range, viz. 0.05–30 μM, detection limit (0.003 μM), reusability (100 times), response time (10s), and stability (4 months). Thus, this work illustrates a simple and novel approach for the development of an improved amperometric enzyme sensor for determination of total phenolic content in fruit juices and alcoholic beverages.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Cuvelier, M.E., Richard, H., Berset, C. (1996). Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. J. Am. Oil Chem. Soc. 73:645–652.
- Lu, Y., Yeap, L.F. (2002). Polyphenolics of salvia-a review. Phytochem. 59:117–140.
- Friedman, M. (1997). Chemistry, biochemistry, and dietary role of potato polyphenols: A review. J. Agric. Food Chem. 45:1523–1540.
- Bosch, F., Font, G., Manes, J. (1987). Ultraviolet spectrophotometric determination of phenols in natural and waste waters with iodine monobromide. Analyst. 112: 1335–1337.
- Mosca, L., De Marco, C., Visioli, F., Cannella, C. (2000). Enzymatic assay for the determination of olive oil polyphenol content: Assay conditions and validation. J. Agric. Food Chem. 48:297–301.
- Bartak, P., Frnkova, P., Cap, L. (2000). Determination of phenols using simultaneous steam distillation-extraction. J. Chromatogr. 867:281–287.
- Ong, C.P., Lee, H.K., Li, S.F. (1989). Optimization of mobile phase composition for high-performance liquid chromatographic analysis of eleven priority substituted phenols. Chromatogr. 464:405–410.
- Angerosa, F., D'Alessandro, N., Konstantinou, P., Di Giacinto, L. (1995). GC-MS evaluation of phenolic compounds in virgin olive oil. J. Agric. Food Chem. 42: 1802–1807.
- Rogers, K.R., Becker, J.Y., Cembrano, J., Chough, S.H. (2001). Viscosity and binder composition effects on tyrosinase-based carbon paste electrode for detection of phenol and catechol. Talanta. 54:1059–1065.
- Jaafar, A., Musa, A., Lee, Y.H., Nadarajah, K., Hamidah, S. (2005). The use of immobilized tyrosinase enzyme in sol-gel film for optical phenol detection. Sains Malaysiana. 34:91–94.
- Jaafar, A., Musa, A., Lee, Y.H., Nadarajah, K., Hamidah, S. (2006). Immobilization of tyrosinase in chitosan film for an optical detection of phenol. Sen. Actuat. B 114:604–609.
- Yaropolov, A.I., Kharubiu, A.N., Emnéus, J., Marko-Varga, G., Gorton, L. (1995). Flow injection analysis of phenol at a graphite electrode modified with co-immobilized laccase and trysoniase. Anal. Chim. Acta. 308:137–144.
- Gommes, S.A.S.S., Rebelo, M.J.F. (2003). A new laccase biosensor for polyphenols determination. Sensors 3:166–175.
- Jaafar, A., Musa, A., Lee, Y.H., Nadarajah, K., Hamidah, S. (2006). Stacked films immobilization of MBTH in nafion/sol-gel silicate and horseradish peroxidase in chitosan for the determination of phenolic compounds. Anal. Bioanal. Chem. 386:285–1292.
- Reku'c, A., Kruczkiewicz, P., Jastrzembska, B., Liesiene, J., Peczyńska-Czoch, W., Bryjak, J. (2008) Laccase immobilization on the tailored cellulose-based Granocel carriers. Ind. J. Biol. Macromol. 42:208–215.
- Silva, C., Araujo, R., Casal, M., Gubitz, G.M., Cavaco-Paulo, A. (2007). Influence of mechanical agitation on cutinases and protease activity towards polyamide substrates. Enz. Microbiol. Technol. 40:1678–1685.
- Mello, L.D., Sotomayor, M.D.P.T., Kubota, L.T. (2003) HRP-based amperometric biosensor for the polyphenols determination in vegetables extract. Sen. Actuat. B 96: 636–645.
- Munteanu, F.D., Lindgren, A., Emneus, J., Gorton, L., Ruzgas, T., Csoregi, E., Ciucu, A., Van Huystee, R.B., Gazaryan, I.G., Lagrimini, L.M. (1998). Bioelectrochemical monitoring of phenols and aromatic amines in flow injection using novel plant peroxidases. Anal. Chem. 70: 2596–2600.
- Jarosz-Wilkolazka, A., Ruzgas, T., Gorton, L. (2004). Use of laccase-modified electrode for amperometric detection of plant flavonoids. Enz. Microb. Tech.,35:238–241.
- Yaropolov, A.I., Kharubiu, A.N., Emnéus, J., Marko-Varga, G., Gorton, L. (1995). Flow injection analysis of phenols at a graphite electrode modified with co-immobilized laccase and trysoniase. Anal. Chim. Acta. 308:137–144.
- Kushwah, B.S., Upadhyaya, S.C., Shukla, S., Sikarwar, A.S., Sengar, R.M.S., Bhadauria, S. (2011). Performance of nanopolyaniline-fungal enzyme based biosensor for water pollution. Adv. Mat. Lett. 2:43–51.
- Navaratne, A.N., Gunawardena, D., Kadawathaarachi, L., Ileperuma, C. (2000). Biosensor approach for monitoring polyphenol oxidase (PPO) activity of selected local fruits with blanching time (steam blanching). Proceedings of the Peradeniya University Research Session Sri Lanka. 12: 72–73.
- Zhang, G., Liu, D., Shaomin, S., Choi Martin, M.F. (2006). Homocysteine biosensor with eggshell membrane as an enzyme immobilization plateform. Sens. Actuat. B: Enz. 114:936–942.
- Tiwari, A., Gong, S. (2009) Electrochemical detection of a breast cancer susceptible gene using cDNA immobilized chitosan-co-polyaniline electrode. Talanta 77:1217–1222.
- Mouryaa, V.K., Inamdara, N.N., Tiwari, A. (2010). Carboxymethyl chitosan and its applications. Adv. Mat. Lett. 1:11–33.
- Yao, Z.A., Wu, H.G. (2010) Characterization of chitosan-chondroitin sulphate blended membranes and effects on the growth of corneal cells. Adv. Mat. Lett. 1:67–74.
- Tiwari, A., Terada, D., Sharma, P. K., Parashar, V., Yoshikawa, C., Pandey, A.C., Kobayashi, H. (2011). An ultra sensitive saccharides detection assay using carboxyl functionalized chitosan containing Gd2O3: Eu3+ nanoparticles probe. Anal. Meth. 3:217–226.
- Carvalho de, A.J.F., Curvelo, A.A.S, Agnelli, J.A.M. (2010). A first insight on composites of thermoplastic starch and kaolin. Carbo. Poly. 82:189–194.
- Rawal, R., Chawla, S.,Dahiya, T., Pundir, C.S. (2011). An electrochemical sulfite biosensor based on gold coated magnetic nanoparticles modified gold electrode. Anal. Bioanal. Chem. DOI 10.1007/s00216-011-5325-4.
- Chawla, S., Rawal, R., Pundir, C.S. (2011). Fabrication of polyphenol biosensor based on laccase immobilized on copper nanoparticles/chitosan/multiwalled carbon nanotubes/polyaniline-modified gold electrode. J. Biotechnol. 156: 39–45.
- Vasdev, K., Kuhad, R.C. (1994). Induction of laccase production in C. bulleri under shaking and static culture conditions. Folia Microbiol. 39:326–330.
- Dhawan, S., Lal, R., Kuhad, R.C. (2003). Ethidium bromide stimulated hyper laccase production from birds nest fungus Cyathus bulleri. Lett. Appl. Microbiol. 36:64–67.
- Fernandes, S.C., de Oliveira, I.R.W.Z., Fatibello-Filho Spinelli, O.A.,Cruz Vieira, I. (2008). Biosensor based on laccase immobilized on microspheres of chitosan crosslinked with tripolyphosphate. Sens. Actuat. B: Chem. 133:202–207.
- Diaconu, M., Litescu, S.C., Radu, G.L. (2010). Laccase-MWCNT-chitosan biosensor. A new tool for total polyphenolic content evaluation from in vitro Sens. Actuat. B: Chem. 145:800–806.
- Jiang, D.S., Long, S.Y. Huang, J., Xiao, H.Y., Zhou, J.Y. (2005). Immobilization of Pycnoporus sangunieus laccase on magnetic chitosan microspheres. Biochem. Eng. J. 25: 15–23.
- Santhiago, M., Vieira, I.C. (2007). Determination in pharmaceutical formulations using a biosensor based on laccase from Aspergillus oryzae. Sens. Actuat. B: Chem. 128:279–285.
- Brondani, D.R., Scheeren, C.W., Dupont, J., Vieira, I.C. (2009). Biosensor based on platinum nanoparticles dispersed in ionic liquid and laccase for determination of adrenaline. Sens. Actuat. B: Chem. 140:252–259.
- Quan, D., Kim, Y., Shin, W. (2004). Characterization of an amperometric laccase electrode covalently immobilized on platinum surface. J. Electroanal. Chem. 561:181–189.
- D'Annibale, A., Stazi, S.R., Vinciguerra, V., Mattia, E.D., Sermann, G.G. (1999). Characterization of immobilized laccase from Lentinula edodes and its use in olive-mill waste water treatment. Pro. Biochem. 34:697–706.
- Corman, M.E., Öztürk, N., Bereli, N., Akgölc, S., Denizli, A. (2010). Preparation of nanoparticles which contains histidine for immobilization of Trametes versicolor laccase. J. Mol. Catal. B: Enz., 63:102–107.
- Liu, Y., Qu, X., Guo, H., Chen, H., Liu, B., Dong, S. (2006). Facile preparation of amperometric laccase biosensor with multifunction based on the matrix of carbon nanotubes-chitosan composite. Biosens. Bioelectron. 21:2195–2201.
- Singh, G., Bhalla, A., Capalash, N., Sharma, P. (2010). Characterization of immobilized laccase from γ-proteobacterium JB: Approach towards the development of biosensor for the detection of phenolic compounds. Ind. J. Sci. Technol. 3:48–53.
- Xu, M., Guo, P., Lu, R., Wang, (2010). Development of amperometric laccase biosensor through immobilizing enzyme in copper-containing ordered mesoporous carbon (Cu- OMC)/chitosan matrix. Mat. Sci. Eng. 30:722–729.
- Abdullah J., Ahmad M., Heng, L.Y., Karuppiah N., Sidek, H. (2007). An optical biosensor based on immobilization of laccase and MBTH in stacked films for the detection of catechol. Sensors. 7:2238–2250.
- Elkaoutit, M., Naranjo-Rodriguez, I., Temsamani, K.R., De La Vega, M.D., Hidalgo-Hidalgo De Cisneros, J.L. (2007). Dual laccase-tyrosinase based sonogel-carbon biosensor for monitoring polyphenols in beers. J. Agri. Food Chem. 55:8011–8018.
- Vianello, F., Ragusa, S., Teresa, M., Cambria, A., Rigo, A. (2006). High sensitivity amperometric biosensor using laccase as biorecognition element. Biosens. Bioelectron. 21:2155–2160.
- Kochana, J., Nowak, P., Jarosz-Wilkołazka, A., Bieroń, M. Tyrosinase/laccase bienzyme biosensor for amperometric determination of phenolic compounds. Microchem. J. 89: 171–174.