Abstract
A new amperometric biosensor was developed for determining hypoxanthine in fish meat. Xanthine oxidase with pyrrole and polyvinylsulphonate was immobilized on the surface of a platinum electrode by electropolymerization. The determination of xanthine-hypoxanthine was performed by means of oxidation of uric acid liberated during the enzyme reaction on the surface of the enzyme electrode at + 0.30V (SCE). The effects of pH, substrate concentration, and temperature on the response of the xanthine-hypoxanthine biosensor were investigated. The linear working range of the enzyme electrode was 1.0 × 10−7 –1.0 × 10−3 M of the hypoxanthine concentration, and the detection limit was 1.0 × 10−7M. The apparent Km(app) and Imax of the immobilized xanthine oxidase were found to be 0.0154 mM and 1.203 μA/mM, respectively. The best pH and temperature value for xanthine oxidase were selected as 7.75 and 25°C, respectively. The sensor was used for the determination of hypoxhantine in fish meat. Results show that the fish degraded very rapidly after seven days and the hypoxanthine amount was found to increase over days of storage.
Introduction
The development of a sensor for xanthine (X) and hypoxanthine (Hx) is of medical and biological importance (Çubukçu et al. Citation2007, Zen et al. Citation2002, Mello et al. Citation2002, Arslan et al. Citation2006a), because the levels of these compounds are generally used in the food industry as an index for evaluating meat or fish freshness (Venugopal Citation2002). The level of hypoxanthine in fish muscle either alone, or in conjunction with the levels of other ATP metabolites, is used as an indicator of quality (Roberts et al. Citation1991, Yano et al. Citation1995). Hx accumulates mostly in the animal muscle; therefore, a close correlation between the nucleotide catabolism and the foodstuff loss of quality can be established (Agüi et al. Citation2006). Thus, the Hx levels are used as an index of fish and meat freshness in the food industry. Methods like chromatography and spectrophotometry are utilized for the detection of X and Hx (Pei and Li Citation2000, Sarker et al. Citation1999). However, since these methods are costly and laborious, alternative systems like amperometric biosensors have been developed for this particular application. These electroanalytical methods are based on the direct oxidation of Hx or its complexes at the electrode surface (Hu et al. Citation1999, Househam et al. Citation1987, Hu et al. Citation2000). In these systems, the level of X and Hx is monitored electrochemically by following H2O2 and/or uric acid oxidation or O2 consumption produced by the xanthine oxidase-catalyzed reaction, depending on the charge of applied potential (Venugopal Citation2002).
Hypoxanthine + O2 → Xanthine + H2O2
Xanthine + O2 → Uric acid + H2O2
Conducting polymers have recently been used as electroactive materials in electrochemical biosensors by virtue of their flexibility in chemical structure and redox characteristics (Lu et al. Citation1995, Kumar and Chen Citation2008, Verghese et al. Citation1998); among the conducting polymers, polypyrole (PPy) has been widely investigated because of its facile polymerization, ease of membrane formation, high conductivity, and chemical stability. Various approaches to hybridize PPy with other materials have been attempted so as to endow the parent PPy with increased mechanical strength for desired applications such as functional electrodes, electrochromic devices and sensors, etc. (Palmisano et al. Citation1997, Ramanathan et al. Citation1995, chaubey et al. Citation2000). The PPy-PVS (polypyrrole-polyvinylsulphonate) composite membrane has been shown to play a unique role as a charge-controllable membrane in which the fixed charges are controlled electrochemically by an internal electronic state (Shimidzu et al. Citation1988).
In this study, uric acid and hydrogen peroxide, produced by catalyzing the oxidation of Hx, are oxidized electrochemically at the same time and cause interference in the reactions. In order to prevent this interference, methylene blue is added to an oxygen-free working atmosphere for getting electrons from xanthine oxidase and preventing the production of hydrogen peroxide (Salaris et al. Citation1991). As a result, xanthine oxidase is immobilized with the entrapment method; after that, uric acid is formed and is oxidized at optimum working conditions. The optimum working conditions were investigated.
Materials and methods
Equipments and reagents
Electrochemical studies were carried out using a BAS 100 B/W electrochemical analyzer and a three-electrode cell. The working electrode was a Pt plate (0.5 cm2). The auxiliary and the reference electrodes were a Pt wire and saturated calomel electrode (SCE), respectively. The pH values of the buffer solutions were measured with a ORION Model 720A pH/ion-meter. Temperature control was achieved with a Grant W14 thermostat. In these experiments, Scanning Electron Microscope JEOL Model 6360 instrument was used.
Xanthine oxidase (100 unit.mL−1) and hypoxanthine were purchased from Sigma. Pyrrole and polyvinylsulphonate were supplied from Fluka and Aldrich, respectively, and all other chemicals were obtained from Sigma. All the solutions were prepared using bi-distilled water.
Preparation of polypyrrole-polyvinylsulphonate (PPy-PVS) composite film
PPy-PVS films were synthesized from an aqueous solution of pre-distilled pyrrole (0.1M) (Aldrich) and the sodium salt of polyvinylsulphonate (25% by weight) (Aldrich) using an electrochemical analyzer with a three-electrode cell (BAS 100 B/W). A Pt plate electrode was used as the working electrode, Pt as the counter electrode, and SCE as the reference electrode. The electrochemical growth of PPy-PVS composite film was carried out by cycling between −1.0 and 2.0 V versus SCE at the scan rate of 50 mV/s (Chaubey et al. Citation2000, Ottero and Olazabal Citation1996).
Immobilization of XOD on polypyrrole-polyvinylsulphonate (PPy-PVS) composite film
The surface of the Pt electrode cleaned according to Gros et al. (Citation2000) was covered with xanthine oxidase and polypyrrole-polyvinylsulphonate by electropolymerization of PPy-PVS. The enzyme, polypyrrole, and polyvinylsulphonate were immobilized by the entrapment method. The electropolymerization was carried out with a cyclic potential scan between- 1.0 and + 2.0 V. The PPy-PVS XOD electrode obtained was rinsed carefully with the corresponding buffer. The XOD enzyme electrode was stored at 4°C in phosphate buffer (pH = 7.75).
Procedure
In order to determine whether the enzyme electrode is sensitive to hypoxanthine, an aqueous buffer solution containing 0.1 M phosphate and 0.1 M sodium perchlorate at a pH of 7.75 was added to the cell. Argon gas was passed through from the solution for ten minutes and a steady-state background current was allowed to decay at a constant value at + 0.30 V. Then the aliquots hypoxanthine stock solution was added to the cell using a micropipette, and argon gas was again passed through for five minutes and stirred for five minutes. The response of the biosensor against xanthine was measured after two to three minutes following the application of a constant potential of + 0.30 V (vs SCE). The cell was treated with argon gas for five minutes prior to each addition of hypoxanthine into the cell. The current values against hypoxanthine concentration were plotted to determine the working range of the electrode.
Preparation of fish extract
Fish samples were purchased from a local market. The determination of Hx in fish meat can be accomplished by the recommended procedure (Watanabe et al. Citation1987). Three to five g of fish sample was homogenized in ca. 15 mL distilled water. The homogeneous solution was then filtered through a membrane filter for the extraction of Hx, and then distilled water was added to the filtrate, producing a total volume of 100 mL of sample solution (Hu et al. Citation2000). Amperometric measurements in sample solutions were performed after transferring the corresponding analytical solution to the electrochemical cell with a potential of 0.3 V versus Ag/AgCl.
Determination of Hx involved three successive additions of samples, which included a known amount of Hx in turn.
Results and discussion
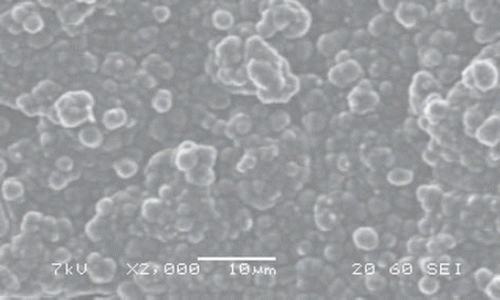
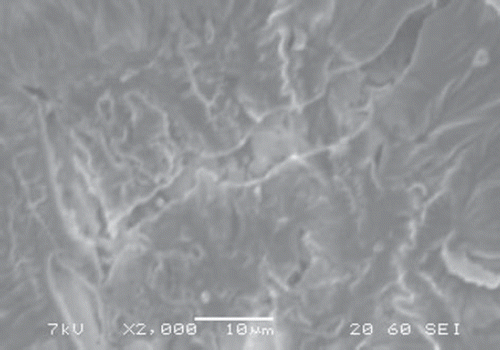
Scanning Electron Microscopy (SEM) of Pt/ PPy-PVS-XOD electrode
SEM picture of Pt/ polypyrrole-polyvinylsulphonate-xanthine oxidase film was investigated. It was observed that the SEM picture of Pt/ polypyrrole-polyvinylsulphonate-xanthine oxidase has a different shape (). This picture, compared with the SEM picture of Pt/polypyrrole-polyvinylsulphonate, shows that the cauliflower-like structure has considerably changed (). This state was attributed to the interaction with xanthine oxidase of the polypyrrole structures.
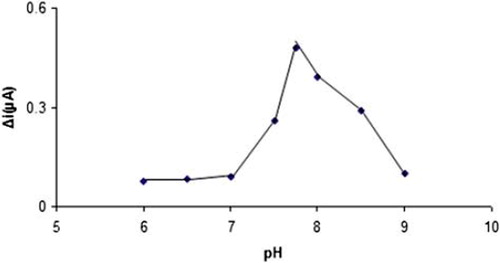
Effect of pH on the activity of the Pt/PPy-PVS-XOD electrode
The effect of pH on the electrode response was investigated by using phosphate buffer systems (0.1 M) between pH 6.0 and 9.0. The effect of solution pH on the xanthine response at the proposed biosensor is shown in . The amperometric response is enhanced by increasing solution pH in the range of pH 7.0 to 7.75 at a constant hypoxanthine concentration of 1.0 × 10−4M. The graph shows that the best pH for xanthine oxidase is 7.75. In xanthine determination with biosensors, pH values other than 7.75 have been employed in the literature (pH 7.0, 7.4, and 8.0). This situation was attributed to the fact that the used polymer and the type of immobilization were different (Arslan et al. Citation2006a, McKenna and Brajter Citation1987, Kilinç et al. Citation1998, Arslan et al. Citation2006b).
Therefore, pH 7.75 is selected by considering both the sensitivity and the stability of the biosensor and consequently further studies have been performed at pH 7.75.
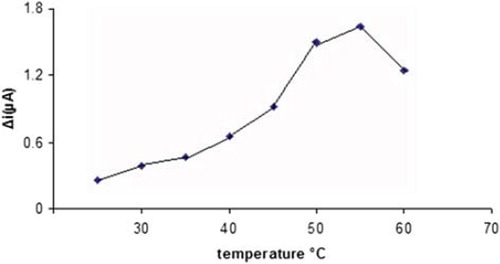
Effect of temperature on the activity of the Pt/PPy-PVS-XOD electrode
The amperometric response of the enzyme electrode to 1.0 × 10−4M hypoxanthine was measured at different temperatures varying from 25 to 60 °C (). The highest current difference was obtained at 50 °C. The current difference increases with temperatures up to 55 °C and decreases afterwards. This decrease can be attributed to the thermal deactivation of the enzyme at the higher temperatures. Moreover, thermal stability of the enzyme at 25, 30, and 35 °C was shown in the literature and it was found that the enzyme was more stable at 25 °C compared to other temperatures. Hence this value was used in all subsequent experiments (Erden et al. Citation2006, Kirgoz et al. Citation2004).
Analytical characteristic
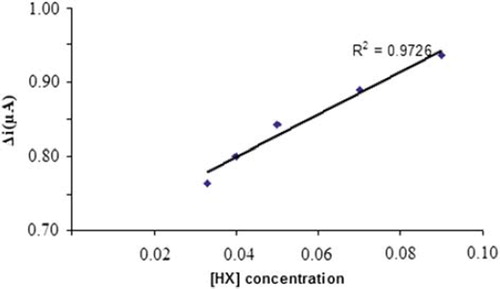
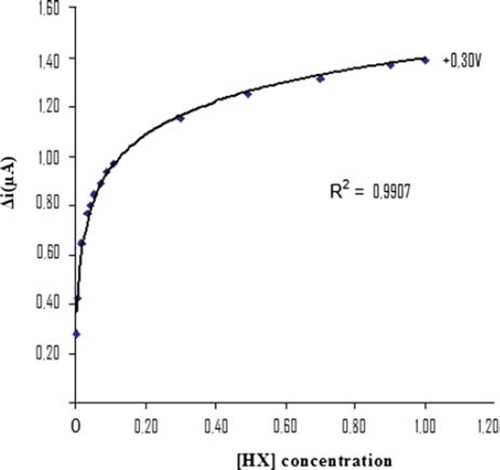
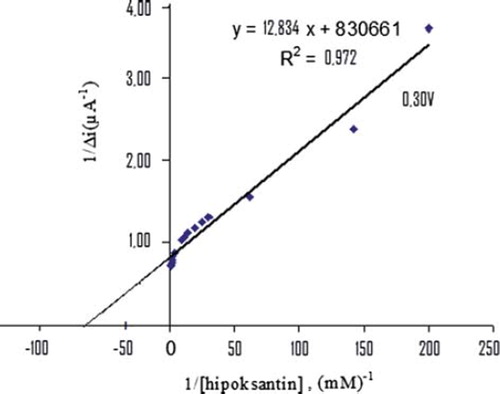
The value of the Michaelis–Menten kinetic parameter (Km), which shows the enzyme–substrate kinetics, was determined by the analysis of the slope of enzymatic reaction. Vmax is the maximum rate for enzymatic reaction. The effect of the substrate concentration on the reaction rate, catalyzed by immobilized xanthine oxidase, was studied using varying initial concentration (1 × 10−7 − 1 × 10−3 M) of Hx (). A linearity for the biosensor was obtained in the concentration range between 1,1x10−4 – 7,0x10−4 M (R2 = 0.972) Hx. The graph for the lower concentration range is given in (Michaelis–Menten plot). It has been shown that the linearity of this graph is highly satisfactory and it can be used for the quantitative determination of hypoxanthine. The linear working range of the enzyme electrode was 1.0x10−7 – 1.0x10−3 M of hypoxanthine concentration and the low detection limit of the biosensor prepared was found to be 1 × 10−7 M. The Km constant, a specific parameter of enzymes, was computed by using the 1/[hypoxanthine]−1/Δi graph given in . (Lineweaver-Burk plot). Km(app) and Vmax values were found to be 0,0154 mM and 1,203 μA/mM, respectively. The Km(app) value that shows the affinity of the biosensor was 0,0154 mM and the values cited in the literature were 1.33 mM, 7mM, 1.11 mM, and 0.04 mM (Arslan et al. Citation2006a, Pei and Li Citation2000, Kilinç et al, Citation1998, Adeloju et al. Citation1996). Since the enzymatic reactions take place through a carrier medium, the affinity of the enzyme towards the substrate is not the same in every carrier media. Therefore, it is normal that Km values differ (CitationArslan et al. 2996a, Çete et al. Citation2007).
Storage Stabilization of Pt/PPy-PVS-XOD electrode
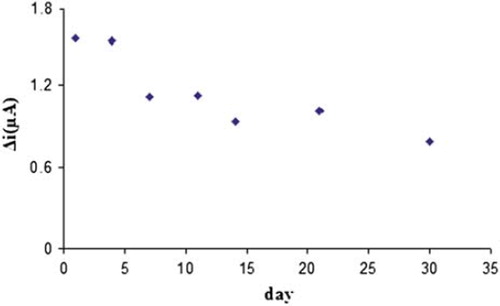
The response of the enzyme electrode prepared under optimum conditions was measured for a period of 30 days at the constant hypoxanthine concentration (1.0 × 10−4 M). The results of seven measurements during this period are plotted in .
There was no noticeable decrease in the response during the first six days but there was a rapid decrease in current values between seventh and tenth day. The electrode showed 49% of the initial amperometric response at the end of the thirtieth day, as can be seen in . This indicated that the biosensor prepared can be used for a week.
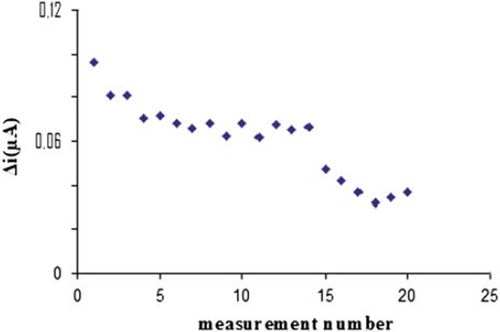
3.6. Reproducibility of the Pt/PPy-PVS-XOD electrode
In order to test the reproducibility of the enzyme electrode prepared, the current changes obtained after subsequent usage were plotted against the number of measurements. The relative standard deviation obtained after 20 measurements at a constant hypoxanthine concentration of 1 × 10−4 M was found as 26%, as shown in .
Sample application
The hypoxanthine (Hx) levels in meat and marine products are important as a food quality control index. The developed sensor was used to measure the Hx in the sample. The results showed that hypoxanthine amount increased over days of storage, as given in . The increase of the hypoxanthine amount was formed by degrading of the purin nucleotide. The hypoxanthine amount was determined by the standard addition method. There was an increase in current values between the first and seventh days. The concentrations of hypoxanthine were found in the first day as 3,125.10−3 M, in the second day as 1,053.10−2 M, in the fifth day as 1,11.10−2 M, and finally in the seventh day as 1,54.10−2 M. When the fish resulted in degradation, the Hx amount increased in fish meat.
Conclusion
A biosensor for the determination of Hx has been developed based on polypyrrole-polyvinylsulphonate. The biosensor has a very low detection limit (1 × 10−6M). It is easy to prepare and highly cost-effective. The biosensor provides a simple and rapid method for the determination of Hx in fish meat.
Acknowledgments
We acknowledge the support of this project by Gazi University Research Fund (FEF 05/2009-23).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adeloju SB, Shaw JS, Wallace GG. 1996. Polypyrrole-based amperometric flow injection biosensor for urea. Analytica Chimica Acta 323:107–113.
- Agüi L, Manso J, Yanez-Sedeno P, Pingarron JM. 2006. Amperometric biosensor for hypoxanthine based on immobilized xanthine oxidase on nanocrystal gold–carbon paste electrodes. Sensors and Actuators B. 113:272–280.
- Arslan F, Kılıç E, Yaşar A. 2006a. An amperometric biosensor for xanthine determination prepared from xanthine oxidase immobilized in polypyrrole film. Artifical Cells Blood Substitutes and Biotechnology 34:113–128.
- Arslan F, Yaşar A, Kılıç E. 20006b. Preparation of Pt/polypyrrole-ferrocen hydrogen peroxide sensitive electrode for the use as a biosensor. Russian Journal of Electrochemistry 42:137–140.
- Çete S, Yaşar A, Arslan F. 2007. Immobilization of uricase upon polypyrrole-ferrocenium film. Artifical Cells Blood Substitutes and Biotechnology 3:607–620.
- Chaubey A, Gerard M, Singhal R, Singh VS, Malhotra BD. 2000. Immobilization of lactate dehydrogenase on electrochemically prepared polypyrrole–polyvinylsulphonate composite films for application to lactate biosensors. Electrochimica Acta. 46:723–729.
- Çubukçu M, Timur S, Anik Ü. 2007. Examination of performance of glassy carbon paste electrode modified with gold nanoparticle and xanthine oxidase for xanthine and hypoxanthine detection. Talanta 74:434–439.
- Erden PE, Pekyardımcı Ş, Kılıç E, Arslan F. 2006. An amperometrik enzyme electrode for creatine determination prepared by the immobilization of creatinase and sarkosine oxidase in poly (vinyl ferrocenium). Artifical Cells Blood Substitutes and Biotechnology 34:223–239.
- Gros P, Durliat H, Comtat M. 2000. Use of polypyrrole film containing Fe(CN)63 − as pseudo-reference electrode: Application for amperometric biosensors. Electrochimica Acta 46:643–650.
- Househam BC, Vandenberg CMG, Riley, JP. 1987. The determination of purines in water using cathodic stripping voltammetry. Analytica Chimica Acta 200:291–303.
- Hu S, Cui D, Mascini M. 1999. Voltammetric detection of nanomolar levels of hypoxanthine in the presence of copper (II) at glassy carbon electrode. Journal of Natural Sciences 4:99–102.
- Hu S, Xu C, Luo J, Luo J, Cui D. 2000. Biosensor for detection of hypoxanthine based on xanthine oxidase immobilized on chemically modified carbon paste electrode. Analytica Chimica Acta 412:55–61.
- Kılınç E, Erdem A, Gökgüneş L, Dalbasti T, Karaoglan M, Ozsoz M. 1998. Buttermilk based cobalt phthalocyanine dispersed ferricyanide mediated amperometric biosensor for the determination of xanthine. Electroanalysis 10:273–275.
- Kirgoz ÜA, Timur S, Wang J, Telefoncu A. 2004. Xanthine oxidase modified glassy carbon paste electrode. Electrochemistry Communications 6:913–916.
- Kumar SA, Chen, SM. 2008. Electroanalysis of NADH using conducting and redox active polymer/carbon nanotubes modified electrodes. Sensors 8:739–766.
- Lu W, Zhao H, Wallace GG. 1995. Pulsed electrochemical detection of proteins using conducting polymer based sensors. Analytica Chimica Acta. 315:27–32.
- McKenna K, Brajter A. 1987. Tetrathiofulvalene tetracyanoquinodimethane xanthine oxidase amperometric electrode for the determination of biological purines. Analytical Chemistry 59:954–958.
- Mello LD, Kubota LT. 2002. Review of the use of biosensors as analytical tools in the food and drink industries. Food Chemistry 7:237–256.
- Ottero TF, Olazabal V. 1996. Electrogeneration of polypyrrole in presence of polyvinylsulphonate: Kinetic study. Electrochimica Acta 41:213–220.
- Palmisano F, De Benedetto GE, Zambonin CG. 1997. Lactate amperometric biosensor based on an electrosynthesized bilayer film with covalently immobilized enzyme. Analyst 122:365–369.
- Pei J, Li XY. 2000. Xanthine and hypoxanthine sensors based on xanthine oxidase immobilized on a CuPtCl6 chemically modified electrode and liquid chromatography electrochemical detection. Analytica Chimica Acta 414:205–213.
- Ramanathan K, Ram MK, Malhotra BD, Murthy, ASN. 1995. Application of polyaniline-Langmuir-Blodgett films as a glucose biosensor. Material Science Engineering C 3:159–163.
- Roberts B, Morris BA, Clifford MN. 1991. Comparison of radioimmunoassay and spectrophotometric analysis for the quantitation of hypoxanthine in fish muscle. Food Chemistry 42:1–17.
- Salaris SC, Babbs CF, Voorhees WD. 1991. Methylene blue as an inhibitor of superoxide generation by xanthine oxidase: A potential new drug for the attenuation of ischemial reperfusion injury. Biochemistry Pharmacology 42:499–506.
- Sarker AK, Ukeda H, Kawana D, Sawamura M. 1999. Spectrophotometric flow-injection analysis for hypoxanthine based on the detection of superoxide anion. Analytical Science 15:1141.
- Shimidzu T, Ohtani A, Honda K. 1988. Charge-controllable poly pyrrole/poly electrolyte composite membranes: Part III. Electrochemical deionization system constructed by anion-exchangeable and cation-exchangeable polypyrrole electrodes. Electroanalytical Chemistry 251:323–337.
- Venugopal V. 2002. Biosensors in fish production and quality control. Biosensors and Bioelectronics 17:147–157.
- Verghese MM, Ramanathan K, Kamlasanan MN, Ashraf SM, Malhotra BD. 1998. Enhanced loading of glucose oxidase on polyaniline films based on anion exchange. Journal Apply Polymer Science 70:1447–1453.
- Watanabe E, Nagumo A, Hoshi M, Konagaya S, Tanaka M. 1987. Microbial sensor for the detection of fish freshness. Journal Food Science 52:592–595.
- Yano Y, Kataho N, Watanable M, Nakamura T, Asona Y. 1995. Evaluation of beef aging by determination of hypoxanthine and xanthine contents: Application of a xanthine sensor. Food Chemistry 52: 439–445.
- Zen JM, Lai YY, Yang HH. 2002. Multianalyte sensor for the simultaneous determination of hypoxanthine, xanthine and uric acid based on a preanodized nontronite-coated screen-printed electrode. Sensors and Actuators B: Chemical 84:237–244.