Abstract
Paraoxonase was covalently immobilized onto a glutaraldehyde containing amino group functionalized chitosan surface by chemical immobilization at pH 8.0. The amount of covalently bound hPON1 was found to be 32 mg/10 chitosan beads. The properties of immobilized enzyme were investigated and compared to those of free enzyme. The effects of various parameters such as pH, temperature, heat, and storage stability on immobilized enzyme were investigated. Kinetic parameters of the immobilized enzyme were also evaluated. Thermal and storage stability experiments were carried out. It was observed that the immobilized enzyme had longer storage stability and retained 50 % of its initial activity during 26 days.
Introduction
Enzymes are biological catalysts that are highly effective and very specific under ambient conditions; therefore enzymatic processes have many industrial applications. General expectations from the commercially used enzyme are efficient use of reactants, maximizing catalytic velocity, and enhancement of the operational lifetime (Kadima and Pickard Citation1990). Use of soluble enzymes has several disadvantages, e.g. instability and sensitivity to process conditions other than the optimal ones (Chibata et al. Citation1978). To improve their economic feasibility in food, pharmaceutical, medical, industrial, and technological processes, soluble enzymes are usually immobilized onto a solid support (Siso et al. Citation1997). For practical purposes, carrier beads with size falling into the millimeter range are mainly used. However, more and more results are being reported on immobilization of enzymes onto microparticles possessing high specific surface area and numerous active sites available for the enzyme molecules to be fixed (Budriene et al. Citation2005). There are a large number of support materials and methods for the immobilization of enzymes. Therfore, it is important that the choice of suitable support materials and immobilization method over the free enzyme should be well justified (Vaillant et al. Citation2000).
In our studies, chitosan – a linear polyglucosamine of high molecular weight obtained by N-deacetylation of chitin in a strong alkali solution – was selected as the support. It has reactive amino (-NH2) and hydroxyl (-OH) groups and has been identified as an ideal support material for enzyme immobilization. It also inexpensive and exhibits high affinity towards protein (Krajewska Citation2004). Chitosan is a natural carbohydrate biopolymer derived from deactylation of chitin. This material is non-toxic, biocompatible, and biodegradable. Over the last several decades, chitinous polymers, especially chitosan, have received increased attention as promising renewable polymeric materials for their extensive applications in the pharmaceutical and biomedical industries, for enzyme immobilization and purification, in chemical plants for wastewater treatment, and in the food industries for food formulations as binding, gelling, thickening, and stabilizing agents (Cho et al. Citation1999, Illanes Citation1994, Ilyina et al. Citation2000, Kumar Citation2000).
Oxidation of low-density lipoprotein (LDL) by redox metals, macrophage, smooth muscle, or endothelial cells in tissue culture modifies its structure so that it binds to the acetyl low-density lipoprotein receptor of monocyte-derived macrophages (Steinberg et al. Citation1989, Steinberg and Witztum Citation1991). It has been suggested that similar events may occur in vivo leading to artherogenesis. Epidemiological evidence has revealed that whereas the LDL concentration in the plasma related directly to the risk of developing coronary heart disease, high-density lipoprotein (HDL) was inversely related to risk (Dawbe 1980). PON1 has recently emerged as the component of HDL most likely to explain its ability to metabolize lipid peroxides and to protect against their accumulation on LDL (Durrington et al. Citation2001). Human serum paraoxonase 1 (PON1), primarily associated with HDL, is a member of a family of enzymes that has the ability to catalyze the hydrolysis of a broad range of carboxyl esters, carbonates, lactones, and toxic organophosphates (La Du Citation1996, Draganov and La Du Citation2004).
Organophosphorous compounds (OP) are widely used as insecticides and unfortunately also as nerve gases (Davies et al. Citation1996). Improving the prophylaxis of OP poisoning is a public health concern that also interests civilian safety officials and the military (Josse and Masson Citation2001). A number of OP insecticides are metabolized by microsomal oxidases to oxygen analogs, the active neurotoxic metabolites which are potent inhibitors of cholinesterase. The oxygen analogs are hydrolyzed by the serum A-esterase, paraoxonase (PON 1), which appears to play a central role in their detoxication and in their toxicity (Costa et al. Citation1999).
There are reports available on the immobilization of enzymes on chitosan-based materials, which deal with activities, stabilities, and reaction kinetics using different enzymes (Krajewska Citation2004, Dutta et al. Citation2002, Tang et al. Citation2007, Cetinus et al. 2009, Gomez et al. Citation2008, Dhananjay and Mulimani 2008). Chang and Juang (Citation2005) reported the immobilization of acid phosphatase on chitosan composite beads and activated clay. Chang and Juang (Citation2005) also reported the immobilization of α-amylase and β-amylase on chitosan–clay composite beads to improve the stability of immobilized enzyme as compared to free enzyme. Altun and Cetinus (2007) reported greater thermal and storage stability in immobilized enzyme on chitosan beads as compared to free enzyme. Prabhu et al. (Citation2009) have reported immobilization of carbonic anhydrase enriched microorganism on different chitosan-based materials.
The immobilization of PON1 on silica gel support has been investigated using a biosensor (Simonian et al. 1999). However, to our knowledge, its immobilization on chitosan has not been studied. The aim of this work is to immobilize the PON1 to chitosan beads so that paraoxonase with variety different enzyme activities could maintain its activity in longer time periods in use in a variety of applications such as chemical synthesis and detoxification of water reservoir. The results showed that chitosan beads can be effectively used as a support for immobilization of the PON1 enzyme.
Experimental
Materials
The materials, including chitosan, Sepharose 4B, L-tyrosine, 9-Aminofenantren, paraoxon, protein assay reagents, and chemicals for electrophoresis and cyanogens bromide, were obtained from Sigma and Merck Chem. Co. All other chemicals were analytic-grade reagents commercially available and used without further purification.
Methods
Preparation of chitosan solution
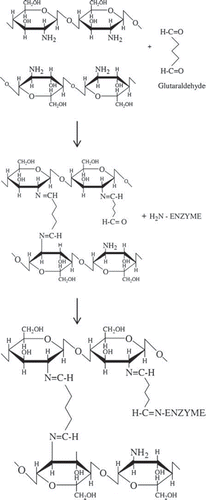
Chiu et al.'s (2004) method was modified for preparation of chitosan beads. Acetic acid (0.5 ml) was added into 50 ml distilled water (% 1 v/v). Chitosan flakes (1.5 g) were added into 50 ml of distilled water and suspended by magnetic stirring for 10 min. (% 3 w/v) Chitosan solution was dissolved in an aqueous solution of acetic acid and mixed continually for 3 h at room temperature. To prepare the coagulation liquid, 26 ml ethanol was added to 100 ml of distilled water (% 26 v/v). Sodium hydroxide (4 g) was added to 100 ml distilled water (1 N NaOH). Ethanol solution was mixed with each at room temperature. The chitosan solution was added drop-wise through a micropipette into a gently stirred coagulation liquid at room temperature. The beads were allowed to cure for 3 h. The obtained beads were filtered and washed with distilled water until neutrality. The beads were added into a cold 0.05 M phosphate buffer (pH 7.0). 5 % glutaraldehyde solutions were carried out with chitosan beads by shaking in a cold phosphate buffer for 4 h, and then the excess of glutaraldehyde was washed out with distilled water.
Purification of paraoxonase from human serum by hydrophobic interaction chromatography
Human serum was isolated from 55 ml fresh human blood and put into a dry tube. Serum paraoxonase was first isolated by ammonium sulphate precipitation (60 - 80%) (Sinan et al. Citation2006). The precipitate was collected by centrifugation at 15.000 rpm for 90 min, and redissolved in a 200 mM tris-HCI buffer. Next, we synthesized the hydrophobic gel, Sepharose 4B-L-tyrosine-9-Aminofenantren, for the purification of human serum paraoxonase (Gençer and Arslan 2009). The column was equilibrated with 0.1 M of a Na2HPO4 buffer (pH: 8.0), including 1 M ammonium sulphate. The paraoxonase was eluted with an ammonium sulphate gradient using 0,1 M Na2HPO4 buffer with and without ammonium sulphate (pH: 8.0).
Immobilization of paraoxonase on chitosan beads
Purified paraoxonase (1 ml) was suspended in chitosan beads (total weight was 1g) so that they were in a 0.02 M cold phosphate buffer (pH: 8.0). The solution was mixed by slight shaking for 16 h at + 4°C. Finally, it was washed with distilled water until free paraoxonase disappeared. Immobilized chitosan beads were kept in a phosphate buffer at + 4°C.
Paraoxonase enzyme assay
PON1 enzyme activity towards paraoxon as a substrate was quantified spectrophotometrically by the method described by Gan et al. (Citation1991). The reaction was followed for 2 min at 37°C by monitoring the appearance of p-nitrophenol at 412 nm on a Biotek automated recording spectrophotometer. The final substrate concentration during enzyme assay was 2 mM, and all rates were determined in duplicate and corrected for the non-enzymatic hydrolysis. A molar extinction coefficient (ε) of 17.100 M −1cm −1 for p-nitrophenol at pH 8.0 in 100 mM Tris–base buffer was used for the calculation. One unit of PON1 activity is defined as 1 μmol of p-nitrophenol formed per minute under the above assay conditions.
Protein determination
Protein concentrations were determined by the Bradford (Citation1976) method using bovine serum albumin as the standard. The amount of bound protein was calculated from the difference between the amount of protein introduced into the reaction mixture and the amount of protein in the filtrate and washing solutions after immobilization.
Optimum pH, temperature, and thermal stability
In order to determine the optimal pH of the enzyme, the enzyme assay was carried out at different pH values (6.0–11.0) at 37°C. Optimal temperature was determined by a standard activity assay in the temperature range from 37 to 65°C.
Kinetic constants of PON1
Determination of Km and Vmax values of free and immobilized enzyme was carried out by measuring activities for both PON1 in the presence of various substrate concentrations (0.05, 0.075, 0.125, 0.250, 0.500, 0.750, and 1 mM). Michael–Menten constant (Km) values and the maximum velocities (Vmax) were determined using the Lineweaver–Burk double reciprocal plot (Lineweaver and Burk Citation1934), in which the reciprocals of the initial velocities of the PON1 activity were plotted against the reciprocals of the concentration of chitosan used.
Results and Discussion
Purification of paraoxonase from human serum and immobilization of enzyme on chitosan
Subsequently, prior to loading onto a hydrophobic interaction column, the precipitate was saturated with 1M ammonium sulphate in order to improve its efficiency for binding to the hydrophobic gel of the column. The hydrophobic gel was synthesized in order to reduce the number of purification steps of the paraoxonase enzyme. The hydrophobic gel was designated based on the retained N-terminal hydrophobic signal peptide for the PON1 enzyme. 9-aminophenantrene, which is a hydrophobic group, was added to sepharose-4B gel matrix with the extension of l-tyrosine arm. PON1 was purified from human serum as indicated in the material methods (Gençer and Arslan 2009). In , it was seen that 526-fold purification were obtained with 60–80% saturation of solid ammonium sulphate and hydrophobic interaction chromatography, respectively. Sinan et al. (Citation2006) reported that PON1 was purified 227-fold. Our purification rate is better than this study. The immobilization procedure was carried out with the purified enzyme.
Table I. Purification of PON 1 enzyme isolated from human serum
The most important criterion for selecting a carrier material for using enzyme immobilization for industrial applications are the stability and cost of the carrier. In this study, the PON1 enzyme was isolated from human serum covalently immobilized on commercially available, inexpensive chitosan. The procedure used for covalent enzyme immobilization on the activated chitosan beads’ surface is shown in .
The specific activity and protein concentration for immobilized enzyme were calculated as 2.93 IU/mg protein and 32 mg/10 chitosan beads, respectively. The immobilization results are summarized in . Karakus and Pekyardımcı (2009) reported that the amount of covalently bound apricot pectinesterase was found to be 1.721 mg/g glass support. Our results are better than this study. Some properties of the immobilized enzyme were investigated and also demonstrated and compared with the free enzyme. Kcat value for the immobilized and free enzyme was determined to be 5.65 and 2.65 EU/mg, respectively (). This result shows that the Kcat value for immobilized enzyme was higher than the free enzyme.
Table II. Immobilization of PON1 from human serum on chitosan beads
Table III. Catalytic efficiency values for free and immobilized PON1
Effect of pH and temperature on PON1 activity
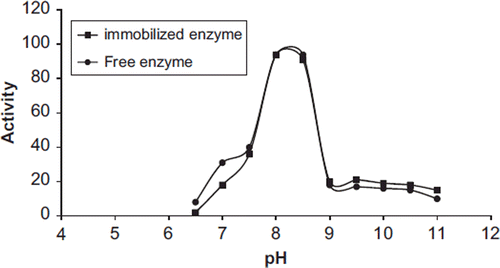
The effect of pH on the activity of free and immobilized PON1 was studied within the pH range of 6.5–11 at room temperature. The enzyme activities obtained are presented in . The maximum activity was observed at pH 8.5 for free PON1 and the immobilized enzyme. Altun et al. (Altun and Cetinus 2007) were determined that the optimum pH of immobilized pepsin enzyme on porous chitosan beads was 3. It may be used for different enzymes. The optimum pH of an enzyme in solution can change, depending on the surface and residual charges of the solid matrix and the nature of the enzyme-bound pH value in the immediate vicinity of the enzyme environment when the same enzyme is immobilized on a solid matrix. The change in the optimum pH normally results in insolubilization of enzymes, depending upon the polymer used as support.
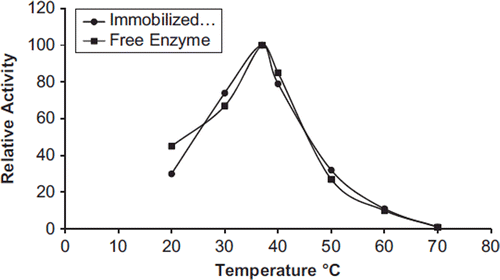
The activities of free and immobilized PON1 were assayed at various temperatures (20–70°C). The effect of temperature on the activity of free and immobilized PON1 is shown in . It was shown that the maximum catalytic activity was obtained at 37°C for free and immobilized enzymes.
The immobilized PON1 retained 30% of its optimum activity, whereas the free PON1 was 25% at 50°C. The free and immobilized PON1were closed active at 70°C.
Thermal stability of free and immobilized PON1
It has been reported that the thermal stability of many enzymes was increased after immobilization on a support because the support material is supposed to preserve the tertiary structure of the enzyme (Arica Citation2000). The authors demonstrated that the thermal stability of enzymes might be drastically increased if they are attached to a complementary surface of a relatively rigid support in a multipoint (Madoery et al. Citation1995, Martinek et al. Citation1977).
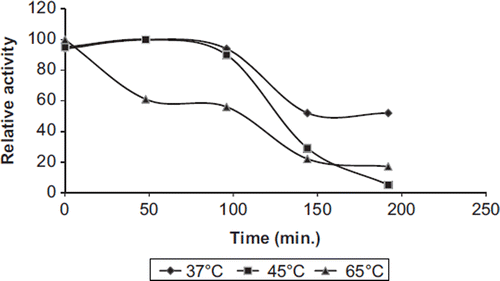
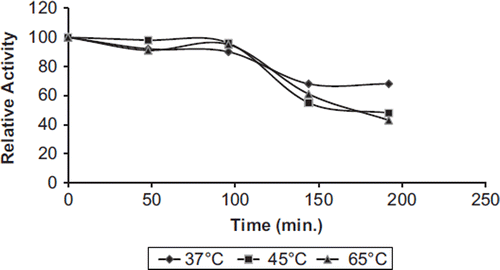
Thermal stability experiments were carried out with free and immobilized enzymes, samples of which were incubated in the absence of substrate at various temperatures. and show the heat inactivation curves between 37 and 65°C for immobilized and free PON1, respectively. During a 200 min incubation period, at 37°C, free and immobilized enzymes retained 50% and 70% activity, respectively. Free and immobilized paraoxonase lost about 40% and 10% of the original activity at 65°C for 45 min, respectively. At all temperatures, the immobilized enzyme inactivated at a much slower rate than the free form.
Free enzymes lost on a large scale their initial activity at 65°C after 200 min. The data obtained from the thermal stability profile were used to analyze some thermodynamic parameters related to human PON1 activity. These results suggest that the thermostability of immobilized enzymes increased after covalent immobilization on chitosan. The activity of the immobilized enzyme, especially in a covalently bound system, is more resistant to heat and denaturing agents than that of the soluble form. If the thermal stability of an enzyme was increased more by immobilization, the potential utility of such enzymes would be extensive (Arica Citation2000).
Enzyme kinetic studies
The kinetic constants (Km and Vmax values) for free and immobilized PON1 enzymes were determined by using paraoxon as a substrate at 37 ± 5°C ().
Km and Vmax values of both enzymes were calculated from the intercepts on x and y axes of the Lineweaver–Burk plots for the free and immobilized PON1, respectively. For the free enzyme Km was 1.067 mM and the apparent Km value of covalently immobilized PON1 was 1.755 mM. The free enzyme Km value was lower than the free enzyme. For the free enzyme Vmax was 125 μmol min −1 mg −1, but upon covalent immobilization of the enzyme on chitosan Vmax increased a little to 181 μmol min −1 mg −1. During the covalent immobilization, structural changes in the enzyme molecule procedure can occur and cause the change in the kinetic parameters of the immobilized enzyme (Arica Citation2000, Madoery et al. Citation1995, Martinek et al. Citation1977). In any case, free PON1 showed lower specificity constants compared to its immobilized counterpart.
Storage stability studies
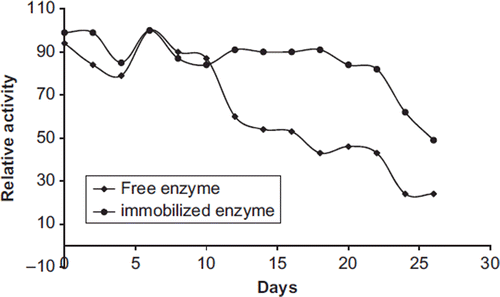
To demonstrate the storage stability of free and immobilized enzymes, the enzyme preparations were stored at 4°C and measured for a period of 30 days. Results are shown in . During the first 10 days, observed activity lost was 15% for both of them. Activities were decreased slowly after 10 days and continued to decrease until day 30. The free enzyme lost about 75% of all its initial activity while the immobilized enzyme retained about 50% of its initial activity after 30 days. The chitosan and the immobilization method provide higher shelf-life compared to that of the free enzyme since the covalent bonds formed between enzyme and support enhance the conformational stability of the immobilized enzyme.
It was reported that the free pectinesterase lost 60% of its initial activity and immobilized enzyme retained 50% of its initial activity within 30 days (Karakus et al. 2008). It was reported that uricase activity after storage for 7 weeks was found to be 42% and 49% of the initial activity values for free and immobilized enzymes, respectively (Çete et al. Citation2007). Kocaturk and Yagar (Citation2010) reported that beads prepared at optimum immobilization conditions were suitable for up to 8 repeated uses. It was reported that Immobilization of lipase was carried out using porous Celite 545 particle as a carrier and the activity of an enzyme was decreased to about a third, when it was used for a second time (Sag roglu 2008). Elcin et al reported that Urease (EC 3.5.1.5) was immobilized within polyanionic carboxymethylcellulose/alginate (CMC/Alg) microspheres coated with a cationic polysaccharide, chitosan. C(CMC/Alg) microspheres showed a nearly stable urease activity of around 80-85% of the initial maximum activity, after the first 100 minutes.
Acknowledgement
The authors most gratefully acknowledge Balikesir University Research Project for the financial support of this work (Project number 2009/02).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Altun GD and Cetinus SA. Immobilization of pepsin on chitosan beads. Food Chem. 2007;100(3):964–971.
- Arica MY. Immobilization of polyphenol oxidase on carboxymethylcellulose hydrogel beads: preparation and characterization. Polym Int. 2000;49(7):775–781.
- Bradford M. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254.
- Budriene S, Gorochovceva N, Romaskevic T, Yugova LV, Miezeliene A, Dieny G, Zubriene A. Beta-galactosidase from Penicillium canescens. Properties and immobilization. Cent Eur J Chem 2005;3: 95–105.
- Çete S, Yasar A, Arslan F. 2007. Immobilization of uricase upon polypyrrole-ferrocenium film. Artif. Cells Blood Subs. and Biotec. 34: 367–380.
- Cetinus SK, Sahin E, and Saraydin D. Preparation of Cu (II) adsorbed chitosan beads for catalase immobilization. Food Chem. 2009;114(3):962–969.
- Chang MY, and Juang RS. 2004. Stability and catalytic kinetics of acid phosphatase immobilized on composite beads of chitosan and activated clay. Proc Biochem. 39 (9):1087–1091.
- Chang MY, and Juang RS. 2005. Activities, stabilities, and reaction kinetics of three free and chitosan-clay composite immobilized enzymes. Enzyme Microb Technol. 36:75–82.
- Chibata I, Tosa T, Sato T, Mori T. 1978. In: Immobilized Enzymes. Research and Development. New York: Wiley & Sons, 2.
- Chiu SH, Chung TW, Gridhar R, Wu WT. 2004. Immobilization of beta-cyclodextrin in chitosan beads for separation of cholesterol from egg yolk. Food Res Int. 37:217–54.
- Cho YW, Cho YN, Chung SH, Yoo G, Ko SW. 1999. Water-soluble chitin as a wound healing accelerator Biomaterials 20:2139–2145.
- Costa LG, Li WF, Richter RJ, Shih DM, Lusis A, Furlong CE. 1999. The role of paraoxonase (PON1) in the detoxication of organophosphates and its human polymorphism. Chem.-Biol. Interact. 119–120:429–438.
- Davies HG, Richter RJ, Keifer M, Broomfield CA, Sowalla J, Furlong CE. 1996. The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat. Genet. 14(3):334–336.
- Dawber TR. 1980. The Framineham Study. The Epidemiology of Atherosclerotic Disease. Cambridge, MA: Harvard University Press.
- Dhananjay SK and Mulimani VH. 2008. Optimization of immobilization process on crab shell chitosan and its application in food processing. J Food Biochem. J Food Biochem. 32:521–535.
- Draganov DI, and La Du BN. 2004. Pharmacogenetics of paraoxonases: a brief review. Naunyn-Schmiedeberg's Arch. Pharmacol. 369:78.
- Durrington PN, Mackness B, Mackness MI. 2001. Paraoxonase and atherosclerosis. Arterioscler Thromb Vasc Biol. 21:473–480.
- Dutta PK, Ravikumar MNV, and Dutta J. 2002. Chitin and chitosan for versatile application. J Macromol Sci. :307–354.
- Elcin AE, Elcin, YM. 2000. Polycation-coated polyanion microspheres of urease for urea hydrolysis Artif. Cells Blood Subs. and Biotec. 28:95–111.
- Gan KN, Smolen A, Eckerson HW, La Du BN. 1991. Purification of Human Serum Paraoxonase Arylesterase-Evidence For One Esterase Catalyzing Both Activities. Drug Metab. Dispos. 19:100–106.
- Gençer N and Arslan O. 2009. Purification human PON1(Q192) and PON1(R192) isoenzymes by hydrophobic interaction chromatography and investigation of the inhibition by metals. J. Chromatography B. 877:134 140.
- Gomez L, Ramirez HL, Cabrera G, Simpson BK, and Villalonga R. 2008. Immobilization of invertase-chitosan conjugate on hyaluronic- acid-modified chitin. J Food Biochem. 32:264–277.
- Illanes A. 1994. Chitin as a Matrix for Enzyme Immobilization. In Advances in Bioprocess Engineering; Galindo E, Ramirez OT, Eds. Dordrecht, Netherlands: Kluwer Academic, 461–466.
- Ilyina A, Tikhonov VE, Albulov AI, Varlamov VP. 2000. Enzymic preparation of acid-free-water-soluble chitosan. Proc. Biochem. 35:563–568.
- Josse D, Masson P. 2001. Human plasma paraoxonase (HuPON1): an anti-atherogenic enzyme with organophosphate hydrolase activity. Ann. Pharmaceutiques Francaises 59(2):108–118.
- Kadima T, Pickard, M. 1990. Immobilization of Chloroperoxidase on Aminopropyl-Glass Appl. Environ. Microbiol. 56(11):3473–3477.
- Karakuş D, Pekyardımcı S. 2009. Immobilization of apricot pectinesterase (Prunus armeniaca L.) on porous glass beads and its characterization. J. Molecular Catalysis B: Enz. 56:13–19.
- Karakuş E, Ozler A, Pekyardımcı S. 2008. Noncovalent Immobilization of Pectinesterase (Prunus armeniaca L.) onto Bentonite Artif. Cells Blood Subs. and Biotec. 36: 535–550.
- Krajewska B. 2004. Enzyme Microb Techol 35:126–39.
- Kocaturk S, Yagar H. 2010. Optimization of polyphenol oxidase immobilization in copper alginate beads. Artif. Cells Blood Subs. and Biotec. 38(3):157–163.
- Kumar MNVR. 2000. React Funct Polym. 46:1–27.
- La Du BN. 1996. Structural and functional diversity of paraoxonases. Nat. Med. 2:1186.
- Lineweaver H, Burk D. 1934. The Determination of Enzyme Dissociation Constants. J. Am. Chem. Soc. 56:658.
- Madoery RR, Gattone CG, Fidelio G. 1995. Bioconversion of Phospholipids by Immobilized Phospholipase A(2). J. Biotechnol. 401:45–153.
- Martinek K, Kilbanov AM, Goldmacher VS, Berezin IV. 1977. The princlipes of enzyme stabilization. Biochimica et Biophysica Acta 4851–4912.
- Prabhu C, Wanjari S, Gawande S, Das S, Labhsetwar N, Kotwal S, Puri AK, Satyanarayana T, and Rayalu S. 2009. Immobilization of carbonic anhydrase enriched microorganism on biopolymer based materials. J Mol Catal B: Enzyme 60:13–21.
- Sagiroglu A. 2008. Conversion of sunflower oil to biodiesel by alcoholysis using immobilized lipase. Artif. Cells Blood Subs. and Biotec. 36: 138–149.
- Simonian AL, DiSioudi BD, and Wild JR. 1999. An enzyme based biosensor for the direct determination of diisopropyl fluorophosphates. Anal. Chem. Acta. 389:189.
- Sinan S, Kockar F, Arslan O. 2006. Novel purification strategy for human PON1 and inhibition of the activity by cephalosporin and aminoglikozide derived antibiotics. Biochimie. 88:565–574.
- Siso MIG, Lang E, Gomez BJ, Becerra M, Espinar FO, Mendez JB. 1997. Enzyme encapsulation on chitosan microbeads. Process Biochem. 32:211–216.
- Steinberg D, Parthasarathy S, Carew TE, Khoo JC, and Witztum JL. 1989. Beyond Cholesterol - Modifications of Low-Density Lipoprotein That Increase Its Atherogenicity. N. Engl. J. Med. 320:915–924.
- Steinberg D, and Witztum JL. 1991. Lipoproteins and Atherogenesis - Current Concepts. Jama-Journal Of The American Medical Association. JAMA 264:3047–3052.
- Tang ZX, Qian JQ, Shi LE. 2007. Characterization of immobilized neutral lipase on chitosan nano-particles. Mater Lett. 61:37–40.
- Vaillant F, Millan A, Millan P, Dornier M, Decloux M, Reynes M. 2000. Co-immobilized pectinlyase and endocellulase on chitin and Nylon supports. Proc. Biochem. 35:989–996.