Abstract
After peripheral nerve injury, axons often project sprouts from the node of Ranvier proximal to the damage site. It is well known that one parent axon can sprout and maintain several regenerating axons. If enough endoneurial tubes in the distal stump are present for the regenerating axons to grow along, then the number of mature myelinated nerve fibers in the distal stump will be greater than the number in the proximal stump. “Multiple regeneration” is used to describe this phenomenon in the peripheral nerve. According to previous studies, a prominent nerve containing many axons can be repaired by the multiple regenerating axons sprouting from another nerve that contains fewer axons. Most peripheral nerves contain a mixture of myelinated motor and sensory axons as well as unmyelinated sensory and autonomic axons. In this study, a multiple regeneration animal model was developed by bridging the proximal common peroneal nerve with the distal common peroneal nerve and the tibial nerve. Differences in the multiple regeneration ratio of motor and sensory nerves were evaluated using histomorphometry one month after ablating the dorsal root ganglion (DRGs) and ventral roots, respectively. The results suggest that the motor nerves have a significantly larger multiple regeneration ratio than the sensory nerves at two different time points.
Introduction
The repairing of peripheral nerve injuries is an important problem in the orthopedic and microsurgical fields. Peripheral nerve regeneration involves the formation of axonal sprouts followed by their outgrowth into regenerating axons and their reconnection with their original targets. One of the most important factors in the correct reinnervation of targets is the precise regeneration of axons from the proximal stump of the injured nerve to the distal nerve stump.
After peripheral nerve injury, the axonal sprouts usually emerge from the first node of Ranvier proximal to the site of damage, traverse the narrow gap of connective tissue between the proximal and distal stumps, and finally enter the endoneurial tubes in the distal nerve stump. Many studies have demonstrated that one parent axon can regenerate and maintain several collaterals in a regenerative distal stump. This process is often referred to as “multiple regeneration.” It has been shown that multiple axonal sprouting occurs during optic nerve regeneration in the goldfish, frog, rat, mouse, and cat; however, the number of regenerating axons observed in addition to the parent axon varies because this research was conducted in different animal models and the regenerating axons were evaluated using different methods (Mackinnon et al. Citation1991, Murray Citation1982, Stelzner and Strauss Citation1986). A previous study demonstrated that one axon can regenerate and maintain up to three or four sprouts in a regenerating rat peripheral nerve. The determining factor for this multiple regeneration appears to be the number of distal endoneurial tubes, which must be greater in the regenerative axons than in the proximal donor axons (Panseri et al. Citation2008, McQuarrie, Citation1985). Results from our previous study in Rhesus monkeys and rats suggest that one proximal stump can be used to repair two distal injured nerves and that a nerve with fewer axons of lesser function can be used to repair a stronger and more important nerve (Zhang et al. Citation2011, Zhao et al. Citation2007). For this peripheral nerve repair method to be successful in a clinical situation, additional studies on multiple regeneration in the peripheral nervous system are needed.
It is well known that peripheral nerves contain myelinated motor and sensory axons as well as unmyelinated sensory and autonomic axons. It remains unknown, however, how many sprouts can be generated from one myelinated motor axon or one myelinated sensory axon. In this study, we calculated the multiple regeneration ratio to determine if myelinated motor axons and myelinated sensory axons generate different numbers of collaterals during regeneration.
Material and Methods
Experimental design
A total of 24 male Sprague-Dawley rats weighing 200–250 g were randomly divided into four groups. All the rats conformed to our multiple regeneration animal model by bridging the proximal common peroneal nerve (PCP) with the distal common peroneal nerve (DCP) and distal tibial nerve (DT). After two months, pure motor and sensory peripheral nerves were collected from two groups by ablating either the DRGs or ventral roots. These rats were sacrificed one month later, and the nerve tissue was collected for morphological evaluation (group names: 3MM and 3MS). The other two groups were subjected to the same treatments as groups 3MM and 3MS, except that sacrificing and tissue collection did not occur until 11 months after the first operation (groups: 1YM and 1YS). The details for these groups are shown in .
Table I. Experiment design.
Surgical techniques
The animals were housed under specific, pathogen-free laboratory conditions with controlled lighting and were given a rodent diet and water ad libitum. Every effort was made to minimize animal suffering and reduce the number of animals used. All the surgical procedures, experimental manipulations, and peri-operative care measures were performed in strict accordance with the Chinese Guidelines for the Care and Use of Laboratory Animals and were approved by the People’s Hospital of Beijing University Medical Ethics Committee. The animals were returned to the animal facility following surgical procedures and monitored for infection, weight loss, and other disabilities.
First surgery (multiple regeneration animal model)
Surgical procedures were performed under a binocular surgical microscope using standard microsurgical techniques. General anesthesia of sodium pentobarbital (30 mg/kg i.p.) was administered by intraperitoneal injection. Anesthetized animals were shaved and prepared, and the surgeries were performed under aseptic conditions. A longitudinal skin incision along the femur was made, and the gluteus maximus muscle was separated from the femur. The sciatic nerve and its two main branches (the common peroneal nerve (CP) and the tibial nerve (TN)) were exposed. The right CP was transected 10 mm distal to the sciatic nerve bifurcation point, and the proximal CPN and TN were separated. The proximal TN was ligated at the bifurcation site and then reversed. The broken end was sewn into the nearby muscle. The proximal CPN was bridged to the distal CPN and TN using a conduit, and 10–0 nylon microsutures were used to fix the epineurial within the conduit. Biodegradable chitin conduits were used in this study (they have been patented by our laboratory and authorized by the State Intellectual Property Office of the People's Republic of China No. ZL01 136314.2; they are artificial nerve grafts consisting of a polysaccharide shell that demonstrates satisfactory biocompatibility and degradation characteristics, and they are now being used in a preclinical study). A 2 mm gap was maintained between the two nerve segments (see and ). The biodegradable chitin conduits were 6 mm long, 0.1 mm thick, and had a 1.5 mm inner diameter. Finally, the muscle incision was sutured, the wound was closed using 4–0 nylon sutures, and the rat was allowed to recover.
Figure 1. Operating microscope photographs (A, C, E, 10 × 5.5) displaying the gross anatomy of the surgical procedures and corresponding schematic drawings (B, D, F). PCP: proximal common peroneal nerve; PT: proximal tibial nerve; DCP: distal common peroneal nerve; DT: distal tibial nerve. A and B demonstrate the surgical procedure for generating the multiple regeneration animal model. The right common peroneal nerve was transected 10 mm distal to the sciatic nerve bifurcation point, and the proximal stump was separated. The PT was ligated at the bifurcate site and reversed. The broken end of the PT was sewn into the nearby muscle. A conduit was used to bridge the PCP with the DCP and DT. A 2 mm gap was left between the proximal and distal stumps. Pictures C, D, E, and F demonstrate the surgical procedure for purifying the peripheral nerve. The lumbar spinous process and lamina were removed to expose the cauda equina. C and D demonstrate the removal of the motor axons by ablating the right L4–6 ventral roots; E and F demonstrate the removal of the sensory axons by ablating the right L4–6 DRGs. The arrows in photo C indicate the right L4–6 ventral roots, and the arrows in photo E indicate the right L4–6 DRGs.
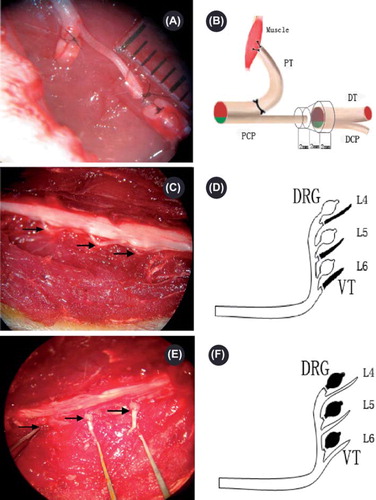
Second surgery (pure motor and sensory peripheral nerve)
General anesthesia was administered following the previously described procedure. Anesthetized animals were shaved and prepared, and the surgeries were performed under aseptic conditions. A posterior midline incision pars lumbalis was made to strip the erector spine, and the lumbar spinous process and lamina were exposed. On either of the two endpoints, the dural sac was carefully opened with microsurgical scissors. The right L4–6 ventral roots were selectively ablated in groups 3MS and 1MS (see and ), and the right L4–6 DRGs were selectively ablated in groups 3MM and 1YM (see and ). Muscles, subcutaneous tissues, and skin were closed accordingly, and the animals were allowed to survive for one month to give sufficient time for the Wallerian degeneration of the sensory and motor axons in the appropriate nerve.
Histological study
One month after the second surgery, the animals were sacrificed with an overdose of sodium pentobarbital by inhalation and perfused through the aorta with 100 mL of saline followed by 500 mL of ice-cold 4% paraformaldehyde (pH 7.4). After perfusion, the right sciatic nerve, including the proximal and distal segments, was removed from each rat. The dissected tissues were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer for 12 h at 4°C. The fixed nerves were rinsed with running water and then rinsed twice in phosphate buffer. Two tissue blocks (approx. 5–6 mm long) were cut, one proximal and one distal to the suture site. Finally, each sample was post-fixed in 1% osmium tetroxide for 12 h, dehydrated through an ethanol series, and embedded in paraffin. Images were collected using an Olympus DP50 camera.
Statistical analysis
Photoshop CS4 and SPSS 15.0. were used. The total number of myelinated axons was counted using the counting tool in the Photoshop CS4 software. The multiple regeneration ratio was calculated by dividing the number of proximal myelinated nerve fibers by the number of distal myelinated nerve fibers. The mean ratio and standard deviation were then calculated for each group. The results are presented as mean ± deviation. Differences in the multiplication ratios between the 3MM group vs. the 3MS group, the 1YM group vs. the 1 YS group, the 3MM group vs. the 1YM group, and the 3MS group vs. the 1YS group were analyzed using a t-test. A P value less than 0.05 was considered statistically significant.
Results
General observations
All of the animals used in this study survived the operations and the desired duration of the experiments. They showed normal eating and drinking behavior, and there were no obvious signs of infection. The right hind limbs and feet of animals in groups 3MM and 1YM showed significant swelling. Autophagy was observed in the right feet of five animals (two and three rats in groups 3MM and 1YM, respectively) after the right L4–6 DRGs were ablated. Animals in which the right L4–6 ventral roots were ablated demonstrated significant denervated muscle atrophy, and no autophagy was observed in these animals.
Histomorphometry
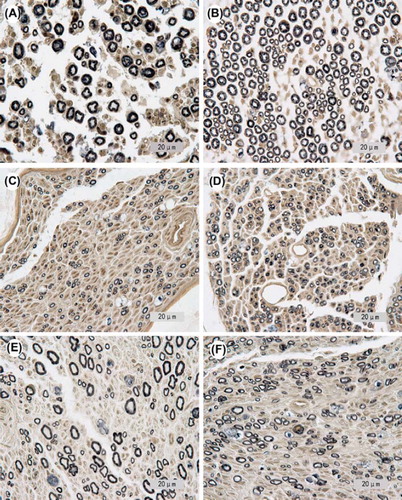
Morphological analysis revealed that the number of sensory nerve fibers is approximately three times greater than the number of motor nerve fibers in the proximal common peroneal nerve. In the proximal stumps, the nerve fibers are evenly distributed, and the diameter of the fibers are similar (see and ). In the 3MM and 3MS groups, an abundant number of myelinated nerve fibers were detected in the distal stumps of both motor and sensory nerves. The nerve fiber diameter and myelin sheath thickness, however, were significantly smaller (see and ). One year after the sleeving surgery, most of the myelin sheaths were more mature. The sheath thickness, nerve fiber diameter, and distribution of the nerve fibers were similar to the proximal stumps (see and ).
Figure 2. Light microscopy images of the proximal common peroneal nerve (A: motor nerve; B: sensory nerve), as well as the multiple regenerated nerves in the distal tibial nerve and common peroneal nerve are shown (C: multiple regenerations for 3MM; D: multiple regenerations for 3MS; E: multiple regenerations for 1YM; F: multiple regenerations for 1 YS). 954×1191 mm (96×96 DPI).
The results of the nerve fiber number analysis are summarized in and . For the 3MM and 3MS groups, the mean motor nerve regeneration ratio is 3.44 (with a standard deviation of 0.26), and the mean sensory nerve regeneration ratio is 2.16 (with a standard deviation of 0.16). For the 1YM and 1YS groups, the mean motor regeneration ratio is 3.07 (with a standard deviation of 0.13), and the mean sensory nerve regeneration ratio is 2.05 (with a standard deviation of 0.14). The nerve regeneration ratios were significantly greater for the motor nerves than for the sensory groups (p = 0.01).
Table II. Number of myelinated nerve fibers in the proximal and distal segments and the multiple regeneration ratio.
Figure 3. A comparison of the different multiple regeneration ratios for the motor and sensory nerves at the two different time points is shown. The ratios for the motor nerves are greater than the ratios for the sensory nerves for both time points. The multiple regeneration ratios for both nerve types at the one-year time point were less than the ratios at the three-month time point. 264 × 161mm (96 × 96 DPI).
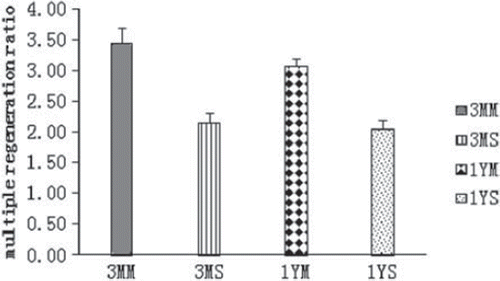
The nerve regeneration ratios for both motor and sensory nerve decreased when the time between the first and second surgery increased. The difference in the multiple regeneration ratios between groups 3MM and 1YM was statistically significant (p = 0.05). There was no statistically significant difference in the multiple regeneration ratios between groups 3MS and 1YS (p = 0.16).
Discussion
It is well known that crushed or severed axons in the peripheral nervous system produce a considerable number of collateral sprouts as they regenerate Murray Citation1982, Stelzner and Strauss Citation1986, Shawe Citation1955, Gutmann and Sanders Citation1943, Sletten et al. Citation2010, Wong and Mattox Citation1991, Horch and Lisney Citation1981, Dunlop Citation2003). Previous studies in rats have focused on the maximum number of collaterals that can be maintained by one axon as the peripheral nerve regenerates, and the estimated maximum value is approximately 3.3 (Jiang et al. Citation2007). Other studies have found that the number of axons regenerating in the distal nerve stumps outnumber the number regenerating in the proximal nerve stumps by as much as to 5:1 (Mackinnon et al. 1981, Zhang et al. Citation2010). These numbers are well established and acknowledged by most anatomists and physiologists. These findings, however, are based on the assumption that neuromas are formed.
Additional studies in Rhesus monkeys have shown that the ulnar nerve can be repaired using the pronator teres nerve from the same upper limbs. After six months, the functional recovery of the nerve was evaluated using morphological and electrophysiological methods. The motor nerve conduction velocity and the number of myelinated nerve fibers suggest that the nerve recovered functionally (Zhang et al. Citation2011). Based on this result, we believe that it may be possible to repair injured nerves using these types of collaterals as donor nerves. We believe that this multiple regeneration capability is a self-repair ability of the peripheral nervous system and may be a new resource of donor nerves to be used in the clinical repair of damaged nerves. Based on previous studies, we may be able to use a small nerve to repair a large nerve trunk. Furthermore, one nerve could be used to simultaneously repair several injured nerves. These types of applications will undoubtedly improve the repair methods currently used for peripheral nerve injury.
Additional questions remain and further studies are still needed. It is well known, for example, that peripheral nerves contain myelinated motor and sensory axons as well as unmyelinated sensory and autonomic axons. The number of sprouts that can be generated from one myelinated motor axon and one myelinated sensory axon, however, remains unknown. In this study, we generated a multiple regeneration experimental paradigm by sleeving the proximal common peroneal nerve with the distal proximal common peroneal and distal tibial nerve. Next, we were able to isolate motor or sensory axons by ablating the DRGs or ventral roots, respectively. The results of the morphological study from both of the time points analyzed indicate that more sprouts can be generated by one motor axon than by one sensory axon.
This number difference may be explained by several factors. First, the neuronal cell bodies of motor and sensory nerves are different. The neuronal cell body is the site of important sub-cellular organelles and of almost all protein synthesis in the cell. Following injury, the neuronal phenotype switches from a transmitting phenotype to a regenerative and repairing phase. The regeneration and repair phase following nerve injury may last for many months. During the early stage of this phase, visible changes in the neuronal cell body include the nucleus returning to the cell center and nucleoproteins reorganizing into compact Nissl granules. During the repair phase, RNA synthesis increases and neurotransmitter synthesis decreases. Sub-cellular organelles are reprogrammed to allow the cell to produce enough proteins and lipids for axon regeneration. Dozens of growth-associated genes, which are normally not expressed in adult neurons, are activated and include neurotrophic factors, transcription factors, molecules participating in axonal transport, and molecules active in the growth cone. Furthermore, trophic support from the denerved target organ also plays an important role in axon regeneration. It is believed that target organs produce soluble protein neurotrophic factors that promote axonal growth. Some of these substances are packaged and conveyed by retrograde transport from the denerved target organs to the neuronal cell bodies by the axon. It has been suggested that denerved target organs release factors that maintain the regenerative phase in the neuronal cell body and promote axon regeneration (Fox et al. Citation2007, Chadaram et al. Citation2007, Gaudet et al. Citation2011). It has been well established that neurons not only require target-derived trophic support for survival, but that they also compete with each other for limited trophic support (Easter et al. Citation1985, Levi-Montalcini Citation1987, Purves et al. Citation1988). These changes in the cell body may occur differently in sensory and motor neurons, which may partially explain the observed differences in their multiple regeneration ratios. The changes in neurotrophic factors produced by the denervated target organs of motor and sensory nerves may also contribute to the differences in the multiple regeneration ratios observed for the sensory and motor nerves. Specific differences in the changes that occur in these two neuronal types, however, have yet to be discovered.
Axoplasmic transport may be another important factor that contributes to the differences in the multiple regeneration ratios. The cell body of an injured neuron must receive accurate and timely information about the site and extent of axonal damage in order to increase its intrinsic growth capacity and activate the expression of regeneration-associated genes. Anterograde transport continuously moves neurotrophic factors from the neuronal cell body to the regenerating axon tips. Retrograde transport moves injury signal molecules from the injury site to the cell body (Grinnell Citation1995). Both fast and slow components of axoplasmic transport supply materials from the cell body to the sites of axon regeneration (Siu Citation2010). Axoplasmic transport occurs in peripheral nerve axons. The normal architecture of motor and sensory nerve axons, however, is significantly different. Motor nerves contain larger axons and associated Schwann cell tubes in addition to a number of smaller fibers. Motor nerves also have a larger fiber width and a smaller fiber density than sensory nerves (Lloyd et al. Citation2007). The results from the myelinated nerve fiber number count in this study also demonstrate that more axons can be counted in a sensory nerve than in a motor nerve. Differences in the speed of and the molecules related to axoplasmic transport in motor and sensory axons may be a result of these architectural differences, and may influence the ability of the regenerating axons to generate multiple sprouts.
A generalized concept in neuronal development that has developed over the past several decades is that neurons require target-derived trophic support for survival (Giehl and Tetzlaff Citation1996, Giehl et al. Citation1997). After peripheral nerve injury, another common outcome is the partial or total loss in neuronal cell bodies, resulting from necrosis and apoptosis (Navarro et al. Citation2007). The predominant reason for this loss is that target organ-derived neurotropic factors cannot be transported back to the cell bodies (Holzbaur and Scherer Citation2011). The published figures for the number of neurons that die after axotomy are highly variable because of the variation in experimental design and the issues regarding the appropriate quantitative methodology (Madison et al. Citation1996, Robinson and Madison Citation2004, Robinson and Madison Citation2005, Uschold et al. Citation2007, Adalbert et al. Citation2006). Currently, it is widely accepted that various factors determine the precise magnitude of cell death and that spinal motor neurons are generally more resistant to cell death than primary sensory neurons (Grinnell Citation1995, Wu et al. 2011, Liuzzi and Tedeschi Citation1991). Some findings even suggest that, after distal nerve injury, small diameter cutaneous neurons express higher levels of caspase-3 and Bax, but lower levels of Bcl-2 than their large diameter muscle afferent counterparts, which express normal levels of caspase-3 and higher levels Bcl-2 (Yoshida et al. Citation2011, Imaizumi et al. Citation2004, Martin Citation2001). Furthermore, the distances from the lesion sites to the neuronal cell bodies were different. Motor neuron cell bodies are located in the anterior horn of the spinal cord gray matter, and primary sensory neuronal cell bodies are located in the dorsal root ganglion. When a peripheral nerve is injured, the distances from the lesion site to the sensory neuronal cell bodies are shorter than the distance to the motor neuronal cell bodies. This difference in distance may cause a difference in the extent of cell body loss after distal nerve injury (Snider et al. Citation1992, Koliatsos and Price Citation1996). The different capabilities of resisting cell body loss after peripheral nerve injury may contribute to the different multiple regeneration ratios observed between motor and sensory nerves.
While the axon segment distal to the injury site degenerates, the Schwann cells that were originally associated with the myelinated axons proliferate, typically within the basal lamina, and form “bands of Bungner” (Shim and Ming Citation2010a). Previous studies have shown that the basal lamina scaffolds of Schwann cells in the distal stump serve as effective pathways for the elongation, maintenance, and maturation of regenerating axons (Shim and Ming Citation2010a). Regenerating axons exhibit a strong preference for growing along the inside portion of the remaining basal lamina tubes in the distal nerve stump. The regenerating peripheral nerve axons enter the basal lamina scaffolds and grow well because the inner surface of the scaffold may contain specific substances that are responsible for supporting the regenerating axons (Gardiner Citation2011, Shim and Ming 2010b). Therefore, it is important that there are enough basal lamina scaffolds to maintain the regenerating axons. Our previous work demonstrated that if enough basal lamina scaffolds can be supplied in the distal stump, then the maximum value of the multiple regeneration ratio of mixed nerves is 3.3 (Jiang 2007). In this study, the number of sensory axons in the proximal stump was approximately two times greater than the number of motor axons, even though the basal lamina tubes supplied in the distal stumps were the same. In sum, a higher number of basal lamina tubes in every parent axon may contribute to the higher multiple regeneration ratio observed for the motor nerves.
The peripheral nervous system is capable of robust regeneration after a nerve lesion. The functional recovery after lesions in the peripheral nervous system, however, requires the accurate regrowth of axons to their original target organs. Previous studies have demonstrated that after femoral nerve transection and repair, motoneurons preferentially reinnervate the quadriceps muscle when given equal access to motor and cutaneous pathways, a process called preferential motor reinnervation (PMR) (Brushart Citation1988, Brushart Citation1993, Brushart et al. Citation1998). During the early stage of regeneration, regenerating motor axons generate multiple collateral sprouts, which randomly reinnervate previous sensory or motor Schwann cell tubes. Regenerating motor axons are directed to sensory target organs when they enter Schwann cell tubes in the distal stump that lead to sensory nerve branches. These axons fail to establish functional contacts and exclude appropriate axons from entering the pathways that they occupy. Eventually, specificity is achieved by pruning collaterals from the sensory pathways and maintaining those collaterals in the motor branch (Brushart Citation1988, Brushart et al. Citation1998, Brushart Citation1990, Kawamura et al. Citation2010). This mechanism for the specificity is described as the “pruning hypothesis.” In this study, the multiple regeneration ratio for motor nerves decreased as the time allowed for regeneration increased. This decrease may be caused by selective “pruning.” Even though the number of regenerating sensory axons is higher than that of regenerating motor axons, the motor nerves generated a higher multiple regeneration ratio than the sensory nerves. The difference in the multiple regeneration ratio may contribute to inaccurate targeting. Taking the higher multiple regeneration ratio for the motor nerves into consideration, we should attempt to increase the number of regenerating sensory nerve axons to balance the ratio. Conversely, improving the number of regenerating motor axons may create more competition with the regenerating sensory axons for the distal basal lamina tubes. Unfortunately, it is unknown which aspect of axon regeneration should be enhanced to improve the accuracy of reinnervation.
Conclusion
The results of this study further demonstrate that motor and sensory axons have different capacities for multiple regeneration after a peripheral nerve injury. A higher number of regenerating sensory axons can be detected in the distal stump because of the higher number of sensory axons located in the proximal stump. The multiple regeneration ratio for motor axons, however, is much higher than the ratio for sensory axons. This finding should be taken into consideration when repairing a peripheral nerve injury, especially when using a smaller nerve to repair a larger nerve or when using one nerve to repair a more prominent nerve.
Acknowledgements
We thank Wang Jing for statistical analysis, and Na Han, Yu-hui Kou, Deng Lei, Zhang Zhenjun, and An Shuai for insightful discussions and for comments on the manuscript.
Declaration of interest
This research project was funded by the Chinese National Natural Science Fund for Outstanding Youth (30625036); Chinese 973 Project Planning (2005CB522604); Chinese National Natural Science Youth Fund (30801169); Beijing City Science & Technology New Star Classification A-2008-10; Chinese National Natural Science Fund (31171150, 81171146, 30971526, 31040043); Chinese Educational Ministry New Century Excellent Talents Support Project (2011).
References
- Adalbert R, Nogradi A, Szabo A, Coleman MP. 2006. The slow Wallerian degeneration gene in vivo protects motor axons but not their cell bodies after avulsion and neonatal axotomy. Eur J Neurosci 24:2163–2168.
- Brushart TM. 1988. Preferential reinnervation of motor nerves by regenerating motor axons. J Neurosci 8:1026–1031.
- Brushart TM. 1990. Preferential motor reinnervation: A sequential double-labeling study. Restor Neurol Neurosci 1:281–287.
- Brushart TM. 1993. Motor axons preferentially reinnervate motor pathways. J Neurosci 13:2730–2738.
- Brushart TM, Gerber J, Kessens P, Chen YG, Royall RM. 1998. Contributions of pathway and neuron to preferential motor reinnervation. J Neurosci 18:8674–8681.
- Chadaram SR, Laskowski MB, Madison RD. 2007. Topographic specificity within membranes of a single muscle detected in vitro. J Neurosci 27:13938–13948.
- Dunlop SA. 2003. Axonal sprouting in the optic nerve is not a prerequisite for successful regeneration. J Comp Neurol 465:319–334.
- Easter SJ, Purves D, Rakic P, Spitzer NC. 1985. The changing view of neural specificity. Science 230:507–511.
- Fox MA, Sanes JR, Borza DB, Eswarakumar VP, Fassler R, Hudson BG, John SW, Ninomiya Y, Pedchenko V, Pfaff SL, Rheault MN, Sado Y, Segal Y, Werle MJ, Umemori H. 2007. Distinct target-derived signals organize formation, maturation, and maintenance of motor nerve terminals. Cell 129:179–193.
- Gardiner NJ. 2011. Integrins and the extracellular matrix: Key mediators of development and regeneration of the sensory nervous system. Dev Neurobiol 71:1054–1072.
- Gaudet AD, Popovich PG, Ramer MS. 2011. Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation 8:110.
- Giehl KM, Schacht CM, Yan Q, Mestres, P. 1997. GDNF is a trophic factor for adult rat corticospinal neurons and promotes their long-term survival after axotomy in vivo. Eur J Neurosci 9:2479–2488.
- Giehl KM, Tetzlaff W. 1996. BDNF and NT-3, but not NGF, prevent axotomy-induced death of rat corticospinal neurons in vivo. Eur J Neurosci 8:1167–1175.
- Grinnell AD. 1995. Dynamics of nerve-muscle interaction in developing and mature neuromuscular junctions. Physiol Rev 75:789–834.
- Gutmann E, Sanders FK. 1943. Recovery of fibre numbers and diameters in the regeneration of peripheral nerves. J Physiol 101: 489–518.
- Holzbaur EL, Scherer SS. 2011. Microtubules, axonal transport, and neuropathy. N Engl J Med 365:2330–2332.
- Horch KW, Lisney SJ. 1981. On the number and nature of regenerating myelinated axons after lesions of cutaneous nerves in the cat. J Physiol 313:275–286.
- Imaizumi K, Benito A, Kiryu-Seo S, Gonzalez V, Inohara N, Lieberman AP, Kiyama H, Nunez G. 2004. Critical role for DP5/Harakiri, a Bcl-2 homology domain 3-only Bcl-2 family member, in axotomy-induced neuronal cell death. J Neurosci 24:3721–3725.
- Jiang BG, Yin XF, Zhang DY, Fu ZG, Zhang HB. 2007. Maximum number of collaterals developed by one axon during peripheral nerve regeneration and the influence of that number on reinnervation effects. Eur Neurol 58:12–20.
- Kawamura DH, Johnson PJ, Moore AM, Magill CK, Hunter DA, Ray WZ, Tung TH, Mackinnon SE. 2010. Matching of motor-sensory modality in the rodent femoral nerve model shows no enhanced effect on peripheral nerve regeneration. Exp Neurol 223:496–504.
- Koliatsos VE, Price DL. 1996. Axotomy as an experimental model of neuronal injury and cell death. Brain Pathol 6:447–465.
- Levi-Montalcini R. 1987. The nerve growth factor 35 years later. Science 237:1154–1162.
- Liuzzi FJ, Tedeschi B. 1991. Peripheral nerve regeneration. Neurosurg Clin N Am 2:31–42.
- Lloyd BM, Luginbuhl RD, Brenner MJ, Rocque BG, Tung TH, Myckatyn TM, Hunter DA, Mackinnon SE, Borschel GH. 2007. Use of motor nerve material in peripheral nerve repair with conduits. Microsurgery 27:138–145.
- Mackinnon SE, Dellon AL, O’Brien JP. 1991. Changes in nerve fiber numbers distal to a nerve repair in the rat sciatic nerve model. Muscle Nerve 14:1116–1122.
- Madison RD, Archibald SJ, Brushart TM. 1996. Reinnervation accuracy of the rat femoral nerve by motor and sensory neurons. J Neurosci 16:5698–5703.
- Martin LJ. 2001. Neuronal cell death in nervous system development, disease, and injury (review). Int J Mol Med 7:455–478.
- McQuarrie IG. 1985. Stages of axonal regeneration following optic nerve crush in goldfish: Contrasting effects of conditioning nerve lesions and intraocular acetoxycycloheximide injections. Brain Res 333:247–253.
- Murray M. 1982. A quantitative study of regenerative sprouting by optic axons in goldfish. J Comp Neurol 209:352–362.
- Navarro X, Vivo M, Valero-Cabre A. 2007. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol 82:163–201.
- Panseri S, Cunha C, Lowery J, Del CU, Taraballi F, Amadio S, Vescovi A, Gelain F. 2008. Electrospun micro- and nanofiber tubes for functional nervous regeneration in sciatic nerve transections. BMC Biotechnol 8:39.
- Purves D, Snider WD, Voyvodic JT. 1988. Trophic regulation of nerve cell morphology and innervation in the autonomic nervous system. Nature 336:123–128.
- Robinson GA, Madison RD. 2004. Motor neurons can preferentially reinnervate cutaneous pathways. Exp Neurol 190:407–413.
- Robinson GA, Madison RD. 2005. Manipulations of the mouse femoral nerve influence the accuracy of pathway reinnervation by motor neurons. Exp Neurol 192:39–45.
- Shawe, GD. 1955. On the number of branches formed by regenerating nerve-fibres. Br J Surg 42:474–488.
- Shim S, Ming GL. 2010a. Roles of channels and receptors in the growth cone during PNS axonal regeneration. Exp Neurol 223:38–44.
- Siu D. 2010. A new way of targeting to treat nerve injury. Int J Neurosci 120:1–10.
- Sletten DM, Weigand SD, Low PA. 2010. Relationship of Q-sweat to quantitative sudomotor axon reflex test (QSART) volumes. Muscle Nerve 41:240–246.
- Snider WD, Elliott JL, Yan Q. 1992. Axotomy-induced neuronal death during development. J Neurobiol 23:1231–1246.
- Stelzner DJ, Strauss JA. 1986. A quantitative analysis of frog optic nerve regeneration: Is retrograde ganglion cell death or collateral axonal loss related to selective reinnervation? J Comp Neurol 245:83–106.
- Uschold T, Robinson GA, Madison RD. 2007. Motor neuron regeneration accuracy: Balancing trophic influences between pathways and end-organs. Exp Neurol 205:250–256.
- Wong BJ, Mattox DE. 1991. Experimental nerve regeneration: A review. Otolaryngol Clin North Am 24:739–752.
- Wu L, Wu J, Chang HH, Havton LA. 2012. Selective plasticity of primary afferent innervation to the dorsal horn and autonomic nuclei following lumbosacral ventral root avulsion and reimplantation in long term studies. Exp Neurol 233:758–766.
- Yoshida N, Kobayashi K, Yu L, Wang S, Na R, Yamamoto S, Noguchi K, Dai Y. 2011. Inhibition of TRPA1 channel activity in sensory neurons by the glial cell line-derived neurotrophic factor family member, artemin. Mol Pain 7:41.
- Zhang P, Kou Y, Yin X, Wang Y, Zhang H, Jiang B. 2011. The experimental research of nerve fibers compensation amplification innervation of ulnar nerve and musculocutaneous nerve in rhesus monkeys. Artif Cells Blood Substit Immobil Biotechnol 39:39–43.
- Zhang C, Zhang P, Wang Y, Yu K, Kou Y, Jiang B. 2010. Early spatiotemporal progress of myelinated nerve fiber regenerating through biological chitin conduit after injury. Artif Cells Blood Substit Immobil Biotechnol 38:103–108.
- Zhao FQ, Zhang PX, Jiang BG. 2007. [Magnifying effect of conduit bridging in number of nerve fibers of broken peripheral nerves: Experiment with rats]. Zhonghua Yi Xue Za Zhi 87:1043–1047.