Abstract
It is necessary to remove residual leukocytes to prevent the blood transfusion-related adverse reactions. This paper describes a facile approach for the surface modification of commercial PBT nonwoven fabrics (PBTNF), used for blood filtration, followed by immobilizing polyvinylpyrrolidone (PVP). The whole blood filtration results revealed that the five types of PBTNF-PVPs’ leucocytes retention rates and erythrocyte recovery rates increased to 96% and 92% compared with the untreated PBTNF. The blood compatibilities results indicated that PVP modified PBTNFs have good blood compatibility, suggesting that PVP-modified PBTNF is a very promising blood filter for selective removal of leukocytes.
Introduction
It has long been reported that, during blood component transfusion therapy, white blood cells (leukocytes) can cause many adverse reactions, including nonhemolytic febrile transfusion reaction (Heddle et al. Citation1999), platelet refractoriness (Eernisse and Brand Citation1981), immunosuppression (Blajchman Citation1997), and transmission of viruses (Brozovic Citation1987). Leucocytes are also known to accelerate the rate of storage lesion. Therefore, removal of leukocytes to low sufficient levels is necessary to prevent undesired reactions, particularly in freshly whole blood. Leukocytes can be removed by using a filter comprised of nonwoven fabric material. Filtration methods have several advantages compared to other methods of removing leukocytes, such as differential centrifugation, sedimentation, cell washing, freezing, and thawing (Treleaven et al. Citation1984).
Currently, different nonwoven polymer fibers such as polyethylene terephthalate (Lewis et al. Citation2003), polybutylece terephthalate (PBT) (Cao et al. Citation2011, Yang et al. Citation2011), and polypropylene (Gerard et al. Citation2011) are used as materials for leukodepletion filters and can be easily processed by melt blowing. The filtration of leukocytes from blood through filters can be regarded as a depth filtration process. Several elementary mechanisms are known to play a role in the retention of particles in depth filters. The mechanisms that are probably involved in leukocyte filtration include blocking or straining, bridging, interception, and adhesion (Bruil et al. Citation1995). Different factors can be responsible for the non-specific adhesion of white cells to surfaces such as the surface chemistry and charge, the wettability, and the morphology of the surface. The specific adhesion could be promoted through the use of selective ligands recognized by the cell receptors (Gerard et al. Citation2011). Clear mechanisms for filtering leukocytes have not been determined. Better understanding of the filtration mechanisms and the properties of blood components will benefit from selectively chosen function groups for leucocytes’ depletion together with minimal losses of other cells than leucocytes and the least side-effects due to the filtration procedure.
In this article, we are interested in the surface modification of PBT nonwoven fabric (PBTNF), the relationship between wettability and leukocyte retention rate, and the functional group modified PBTNF's blood compatibility. Surface wettability is thought to be an important factor affecting blood compatibility (CitationHoffman). Furthermore, a good balance between hydrophobicity and hydrophilicity characteristics is required for excellent blood compatibility (Ratner Citation2004, El-Rehim and El-Arnaouty Citation2004). Different methods have been reported to modify the wettability or the hydrophilicity of nonwoven fabric surfaces in order to improve the blood compatibility. The most usual methods are plasma (Kim et al. Citation2009), UV (Yang et al. Citation2010), and radiation processes (El- Rehim and El-Arnaouty Citation2004). Here, we used the oxygen plasma method to activate the PBTNF surface in order to react with the hydrophilic monomers.
The polyvinylpyrrolidone (PVP), which is a hydrophilic polymer with no hydroxide group or ionic charged one, is known for good blood compatibility. The polysulfone membranes are now used as hemodialysis membranes in the medical industry (Higuchi et al. Citation2002) are blended with PVP. Higuchi et al. (Citation2002) reported that PVP were covalently conjugated on the surface of polysulfone membrane with a multiple chemical process. The PVP-modified polysulfone membrane gave lower protein adsorption from a plasma solution and a more suppressed number of adhered platelets than the original polysulfone. Robinson et al. (Robinson and Williams Citation2002) reported that PVP could be simply adsorbed on silica particles to inhibit protein adsorption and even to remove adsorbed proteins from its surface. The principal reason for PVP applications is their excellent biocompatibility with living tissues and extremely low cytotoxicity.
The purpose of this study was to increase leukocyte retention and erythrocyte recovery rates through surface modification using PVP that was grafted by a plasma grafting method. Different parameters have been studied, including the effect of the wettability of PBTNF on the retention of the blood cells, the morphology of blood cells after filtration, the free hemoglobin in whole blood before and after filtration, and the blood compatibility. Lastly, blood filtration experiments have been performed on the laboratory scale.
Materials and Methods
Materials
Commercial PBTNF (0.40–0.50 mm of thickness, 90 g/㎡, fiber diameter: 2.5–5.0 μm) were bought from Shanghai ShiLong Company (China). For the leukodepletion application, an oxygen-plasma treatment was performed (PS-PJ, AST Products Inc., USA). 1-vinyl-2-pyrrolidinone was bought from Sigma-Aldrich. Acetone, acetic acid, and ethanol were purchased from Xilong Chemical Reagent Company. Fresh health acid citrate dextrose solution anticoagulant (ACD) human blood was a gift from Chengdu Blood Center. Water was obtained with a Pall cascada bio-water Purification System. All chemicals were used without further purification.
O2 plasma treatment and PVP graft reaction
PBTNF was washed in ethyl alcohol by an ultrasonic washing machine and dried at 70°C for 4 h. PBTNF was treated using oxygen plasma and placed on a stainless-steel sample holder. The pressure in the bell jar was reduced to 0.1 torr, followed by introduction of oxygen into the bell jar at a flow rate of 80 sccm (Kim et al. Citation2009). In order to achieve different hydrophilicity PBNF-PVP, we changed the plasma treatment machine power (200, 100, 50, 25, 15w, respectively). The abbreviated names of the five PVP modified PBTNF were PBTNF-P15, PBTNF-P25, PBTNF-P50, PBTNF-P100, and PBTNF-P200, respectively. The O2 plasma-treated PBTNF was immersed in 15 vt% aqueous 1–vinyl–2–pyrrolidinone solution and incubated at 80°C for 3 h. After graft polymerization, PBTNF were washed in acetone using an ultrasonic bath for 30 min and then washed with bulk distilled water with ultrasound for 2 h. The washed PBTNF was dried for 4 h at 70°C to complete the preparation of PVP-grafted PBTNF.
Surface characterization
X-ray Photoelectron Spectroscopy (XPS) spectra were recorded using a Kratos XSAM800 spectrometer with an Al Kα monochromatic X-ray source. All survey and core-level (high-resolution) spectra were referenced to the C 1s hydrocarbon peak at 284.8 eV in order to compensate for surface charging effect. The pressure in the analysis chamber was maintained at 2 × 10− Pa or lower during each measurement.
Evaluation of hydrophilicity of PBTNF
The determination of critical wetting surface tension (CWST) of the original and modified PBTNF was determined using a series of standard solutions having a range of surface tensions (Yang et al. Citation2010, Lewis et al. Citation2000, Natori et al. Citation2006). Ten drops (each drop was 10 μL) of the standard solutions were placed independently on representative portions of the fabric. Observations were made after 10 min to determine whether or not each drop had completely wetted the surface. Wetting was defined as the absorption of at least nine of the ten drops by the test sample within 10 min. The CWST of the test sample was defined as the highest surface tension of the standard solutions which wetted the sample. All samples were repeated three times.
Blood filtration
Device
An acrylonitrile butadiene styrene filter holder (Model 40mm; Chengdu Shuanglu Medical Apparatus & Instruments Co., Ltd., China) was used to filter 50 mL whole blood. Surface-modified PBTNFs (diameter: 28 mm) were placed inside the filter holder and the nonwoven filter layer thickness with six layers. Then the filters were assembled with ultrasonic plastic welders (Shenzhen Kexin Ultrasonic Equipment Company, China). The distance between the top of the blood bag and the filter outlet was 40 cm and the flow rate was controlled by gravity.
The filters were evaluated with respect to leukocyte retention, erythrocyte recovery, and platelet retention rate. Untreated PBTNF was used as a control in the filtration experiments. Whole blood was obtained from the Chengdu Blood Center on the condition that it was used only for research purposes.
Leukocyte retention, erythrocyte and platelet recovery
The numbers of erythrocytes (or RBCs) and platelets (or PLTs) before and after filtration were measured using an automatic blood cell counter (BC3200; Shenzhen Mindray Company, China). The leukocytes after filtration were measured using Nageotte Brightline Hemacytometer (Haussser Scientific Horsham, USA) (Cassens et al. Citation2002, Moroff et al. Citation1994, Dzik et al. Citation2000, Dijkstra et al. Citation2004). Nageotte counts were performed as described. We diluted 100 μL of whole blood in 900 μL of Turk solution (1-in-10 dilution) containing 0.01% (w/v) aqueous gentian violet (Xilong Chemical Reagent Company) and 1% (v/v) glacial acetic acid (Xilong Chemical Reagent Company). After 10 minutes’ rest, the diluted sample was put on a large-volume (50 μL) Nageotte chamber and the WBC's concentration was evaluated by counting the WBCs within the chamber using a conventional white light microscope (Leica DM2000, Germany) with a 20× objective lens. The surplus WBCs’ concentration, leukocyte retention, erythrocyte recovery, and platelet retention rate were calculated using the following equations.
Amounts of free hemoglobin before and after filtration
The free hemoglobin (FHb) was determined by chemical method (Administration Citation2009). The whole blood before and after filtration was centrifuged for 20 min at 2000 g centrifugal force to obtain plasma. 20 μL plasma was added into a tube, then 1 mL 3, 3’- dimethyldiphenylamine (0.25 g 3, 3’- dimethyldiphenylamine resolved in 90 mL acetic acid, and the solution was diluted to 100 mL), and 1 mL fresh H2O2 (1%, V:V) were also added into the tube, respectively. After mixture, the solution was motionless for about 10 minutes. 10 mL acetic acid (10%, V:V) was added into the above solution in order to stop the reaction. Lastly, the amount of FHb was determined immediately by spectrophotometer (Beckman DU800 UV-visible spectrophotometer) at 435 nm. The standard FHb was made according to the Chinese standard (Administration Citation2009). All samples were repeated three times.
Morphology of blood cells after filtration
After filtration, the PBTNFs (1 cm × 1 cm) were gently washed with iso-osmia PBS and fixed in 2.5% (v/v) glutaraldehyde in PBS (1 mL) for 2 h at 4°C. The PBTNFs were slowly dehydrated by successive immersions in aqueous solutions of 30, 75, 90, 95, 100, and 100% v/v ethanol and dried in a vacuum freeze-dryer (FD-1, Shanghai BiLang Instrument Company). Samples were sputter-coated with gold. Subsequently, the samples and morphologies of the blood cells were examined using a scanning electron microscope (SEM). SEM images were obtained using a wolfram SEM instrument (JSM-6510, JEOL, Japan) after the samples were coated with a 20 nm gold layer.
Evaluation of blood compatibility
Hemolysis assay
Hemolysis assay was performed to evaluate the blood compatibility of original and PVP-modified PBTNF. The PBTNF was cut into pieces of 1 cm × 1 cm before being transferred into polystyrene tubes. An amount of 10 mL of 0.9% normal saline was poured into each of the tubes and kept at 37°C in a shaking water bath. After 60 min incubation, 200 μL of diluted ACD blood (8 mL of ACD blood was diluted with 10 mL of normal saline) was dropped into each of the tubes. All of the tubes were further incubated at 37°C for 60 min. Similarly, positive and negative controls were produced in separate tubes by adding 200 μL of the diluted blood to 10 mL of distilled water and normal saline, respectively. The fluid was then transferred into a fresh polystyrene tube and centrifuged at 2500 rpm for 5 min. The absorbance of supernatants was measured at 545 nm using a spectrophotometer (Beckman DU800 UV-visible spectrophotometer). The hemolysis ratio (HR) was calculated according to the following formula:
Where As, Apc, and Anc are the absorbance of sample supernatant, positive control, and negative control, respectively (Fang et al. Citation2004).
Complement activation
The amounts of human complement fragment 3a (C3a) and 5a (C5a) before and after filtration were measured using the ELISA method. The whole blood before and after filtration were centrifuged for 20 min at 2000 g (4°C) centrifugal force to obtain plasma. Then the plasma were kept in −20°C immediately. The frozen plasma were melted at 37°C when we did the ELISA experiments. The C3a and C5a ELISA Kits were produced by CUSABIO Biotech Co., Ltd., China.
Results
XPS
The chemical compositions of the original and PBTNF-P200 calculated from the XPS spectra are shown in . The oxygen content (17.34%) of the PBTNF was increased with PVP grafting (24.26%). The nitrogen content was observed on the surface of PBTNF-PVP. Through the high-resolution C1s spectra of PBTNF and PBTNF-P200 (), the XPS C1s components of the surface-modified PBTNFs were calculated as shown in . For PBTNF-P200, the peak at 287.4 eV increased whereas the peak at 288.9 eV decreased due to the content of the amide bond (CONH) in immobilized PVP. In short, the above results clearly demonstrated that PVP was grafted onto the PBTNF surface successfully.
Table I. Chemical composition of original and PVP-modified PBTNFs from XPS.
Figure 1. XPS high-resolution C-1 spectra of original PBTNF (a) and PBTNF-P200 (b); 209 × 297 mm (300 × 300 DPI).
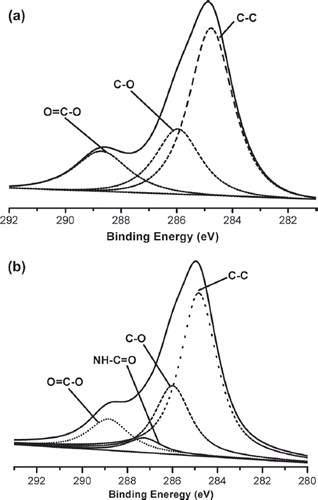
Table II. High-resolution XPS C-1 data for original and PVP modified PBTNFs.
Wettability control of filter materials
The surface wettability of filter materials was influenced greatly by the type and the amount of monomer grafted onto original PBTNF. The amount of grafted monomer was influenced by many factors such as process time, monomer concentration, power, and the amount of gas flow time. The amount of PVP immobilized on the PBTNF was hard to determine. In order to facilitate control of the amount of grafted monomer, the plasma treatment time, the gas flow time, the monomer concentration, and the reaction time were fixed, but only the power changed. Untreated PBTNF cannot be wetted by water, since its CWST was 44.2 × 10−3N · m−1 and the surface tension of water was 72.8 × 10−3N · m−1 at 25°C. As shown in , the surface wettability of modified PBTNF was significantly improved by grafting monomers with PVP.
Table III. The surface CWST of PBTNFs.
Filtration results
Leukocyte retention, erythrocyte recovery and platelet retention rates
All the apparatuses were filtered with whole blood 50 mL in order to confirm leukocyte retention as well as the erythrocyte recovery and platelet retention rate. In order to compare the leukocyte removal efficiency, the whole blood was over-used. The results of the original PBTNF were used as a control. The leukocyte retention rates of PVP modified PBTNF were significantly improved as the wettability was increased (see ).
Figure 2. Leukocyte retention, erythrocyte recovery, and platelet retention rates; 289 × 200 mm (300 × 300 DPI).
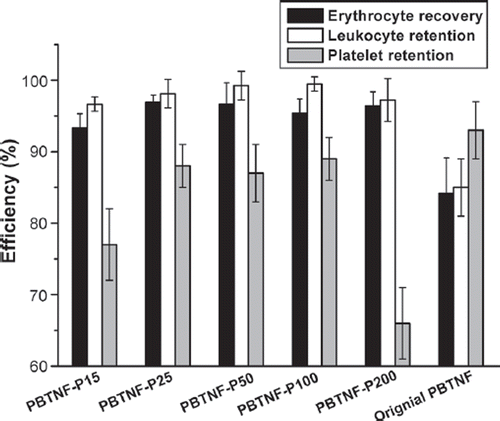
When PBTNF-P100 was filtering with one unit (200 mL) of whole blood, PBTNF-P100 exhibited superior leukocyte retention rates (99.5%) and good erythrocyte recovery rate (98.5%). These results were similar to commercial filters such as Pall and Asahi products (Kim et al. Citation2009).
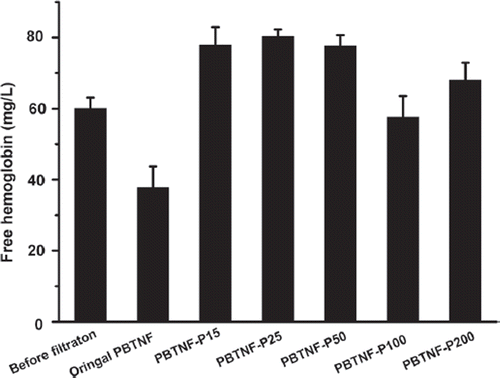
Amounts of free hemoglobin before and after filtration
Transfused blood contains free hemoglobin (FHb), as well as fragile erythrocytes that produce additional FHb. Transfusing blood which contains massive free hemoglobin has deleterious effects on organ function including the kidney (Nishiyama and Hanaoka Citation2000). The Chinese Medical Vocation Standard “YY0329-2009” leukocyte reduction filters for single-use claims that the free hemoglobin should be less than 300 mg/L after filtration 400 mL whole blood or 200 mL red blood cell concentrates. From the , all five types of PBTNF-PVP’s FHb were less than 80 mg/L, which was consistent with the Chinese Medical Vocation Standard “YY0329-2009” leukocyte reduction filters for single use. What's more, after seven days’ storage, the FHb of blood filtered by the original PBTNF was increased to 215 ± 20 mg/L, while those of PVP modified PBTNF were about 100 mg/L. Those results also revealed that the PVP-modified PBTNF had good compatibility with erythrocyte.
Morphology of blood cells after filtration
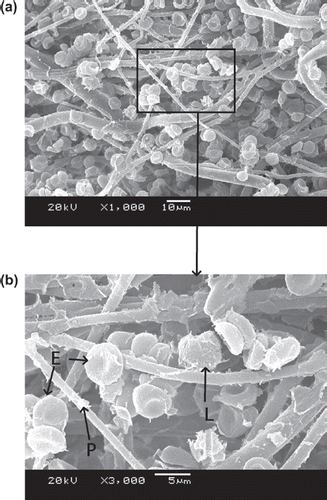
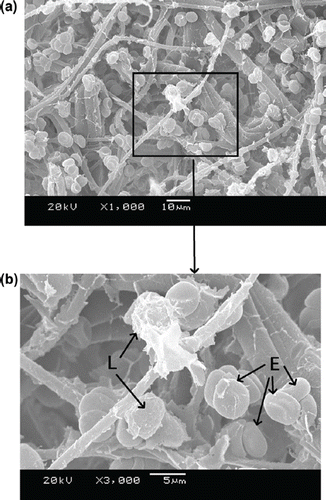
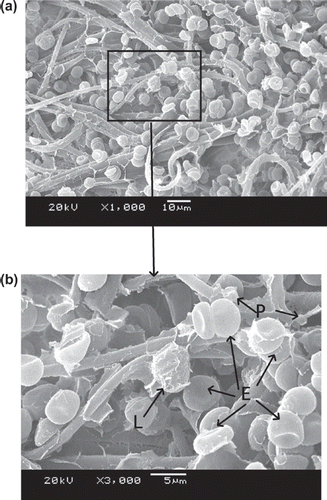
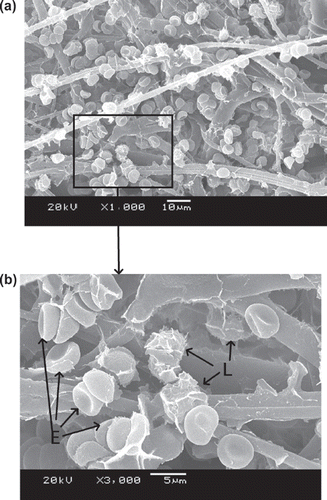
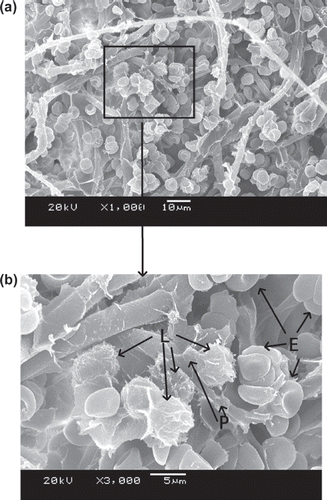
–9 show scanning electron microscope (SEM) microphotographs for original PBTNF and PBTNF-PVP after filtration. Filtered blood cells were found in the upper layer of PBTNF (–9). In terms of morphology, erythrocytes and leukocytes have very different shapes; erythrocytes have a donut-like shape with a hump and leukocytes have protruding crests. SEM of the filter surface after whole blood contact revealed that the platelet adhesion and protein adhesion were reduced as the wettability was improved. Platelets were observed on occasion (P).
Blood compatibility
Hemolysis assay
Hemolysis ratio (HR) was adopted to evaluate the erythrocyte compatibility of filter materials. Also, in our experimental results, the HR of origin PBTNF, PBTNF-P15, PBTNF-P25, PBTNF-P50, PBTNF-P100, and PBTNF-P200 were about 1.00% ± 0.50%, 0.30% ± 0.32%, 0.25% ± 0.22%, 0.31% ± 0.10%, and 0.45% ± 0.50%. This implies that the five types PBTNF-PVP all are compatible with erythrocyte.
Complement activation
C3a and C5a anaphylatoxins are cytokine-like polypeptides generated during complement (C) system activation and released at the inflammatory site. C3a and C5a can cause an allergic response when this kind of blood (containing a lot of C3a and C5a) was transfused into patients. Evidence of complement activation was obtained by measuring fluid phase C3a and C5a. This assay was used as a way to characterize the blood compatibility of PVP surface-modified PBTNF. The original PBNTF and five types of PBTNF-PVP were all elevated in this experiment. From , there were no detectable differences within the C3a results, while there were differences in the C5a results. The C5a were reduced after filtration, possibly due to the adherence of C5a on PBTNF surface.
Table IV. The results of C3a and C5a determination.
Discussion
In this study, PBTNF was treated using a surface-modification method with PVP for removal of leukocytes from blood components. PVP was graft-polymerized onto the surface of PBTNF after oxygen plasma treatment was conducted. The hydrophilicity of the PBTNF surface was improved greatly by covalent immobilization of PVP. A. Lewis et al. (Citation2003) have reported that the blood filtration materials whose CWST > 78 m N · m−1 have good hemocompatibility. We prepared five types of PBTNF-PVP with different wettability to investigate the leukocyte filter efficiency and blood compatibility.
All five types of PVP-modified PBTNF had higher leukocyte-removing efficiency in comparison with the original PBTNF. Other researchers (Bruil et al. Citation1994, Lim and Cooper Citation1991, Neumann et al. Citation1979) also had found similar features about the relationship between the leukocyte adhesion and the wettability. To explain the increase of leukocyte adhesion to surfaces with increased wettability, Neumann et al. (Citation1979) used a thermodynamic model in which the change in free energy resulting from adhesion was related to the surface-free energy of the solid surface, which in turn was related to the surface wettability.
At the same time, the erythrocyte recoveries for each type of PBTNF-PVP were also increased after the wettability was improved compared with the original PBTNF. However, all five types of PBTNF-PVPs’ platelet retention rates were still high. We should consider the removal of the platelet from two sides. On the one hand, we should remove the platelet as much as we can. The reason is that the platelet surface also has human leukocyte antigen (HLA), which can cause HLA all immunization (Murphy et al. Citation1986). On the other hand, we should keep the platelets as much as we can, if we want to separate platelets from whole blood after filtration. However, the current commercial leukocytes can hardly do it. If the platelets will be needed, the leukocytes’ filter should be used after the platelets were already separated. The leukocytes’ mechanisms have been debated (Bruil et al. Citation1995). There is evidence that platelets’ adhesion can promote subsequent adhesion of leukocytes. Garvin (Citation1961) and Rasp et al. (Citation1981) have reported that the leukocytes’ adherence on polymer was dependent on the prior adherence of platelets. It also was reported that activated platelets can release adhesive proteins such as fibrinogen, which may promote leukocyte adhesion (Hirafuji and Shinoda Citation1991). This theory can be used to explain why it is hard to remove 95% of the leukocyte in warm blood (just collected from human body). If the warm blood was cooled to 4°C (the platelet would be activated at low temperature), the leukocyte retention rate would increase significantly.
Three mechanisms of leucocytes entrapment could be recognized through –9 by SEM. Firstly, indirect adhesion: adhesion and spreading of activated platelets to the filter material of granulocytes to the platelets (Steneker et al. Citation1992). In , some platelets were clearly aggregated on the surface of proteins and some of them did not maintain their shape, which indicated that the platelets were activated. The leukocytes were sticking on the activated platelets’ surface. This phenomenon also can be evidence that platelets’ adhesion and activation can promote subsequent adhesion of leukocytes. That's why the original PBTNF has good leukocyte retention rates (around 82%). Secondly, direct adhesion: small pseudopods of leukocytes to surrounding the fibers. The small pseudopods of leukocyte adhered on the fibers can be observed in and . Lastly, mechanical sieving: leucocytes were caught in the pores formed by the fibers, and blocked by each other when reaching a pore or intercepted in dead ends. This sieving phenomenon can also be observed in and .
It has been generally accepted that hydrophilic materials are less sensitive to protein adsorption than hydrophobic ones, the principle behind which is that hydrophilic surfaces preferentially adsorb water rather than solutes, leaving the membrane surface with protein resistance (Mockel et al. Citation1999). Leukocyte adhesion to surfaces is largely influenced by preadsorbed proteins. It is known that albumin has an inhibitory effect on the adhesion of leukocytes to solid surfaces, whereas globulins enhance the adhesion of leukocytes (Bruil 1995). In , the platelet adhesion and protein adsorption of PBTNF-15, PBTNF-25, PBTNF-50 PBTNF-P100, and PBTNF-P200 were less than the original PBTNF. That may be another reason why the original PBTNF has good leukocyte retention rates (> 82%). From the results of platelets retention rates, the platelet adhesion did not reduce significantly as the wettability was improved. The reason may be due to the plasma treatments of material surface depth being too low, causing the inside of the PBTNF to be hydrophobic.
The results of FHb before and after filtration revealed that the PBTNF-PVP had good compatibility with erythrocytes. Also, the HR results of five types of PBTNF-PVP were very low, which also means they are compatible with erythrocytes. All the C3a and C5a results imply that the original PBTNF and five types of PBTNF-PVP may have no influence on complement activation. Complement activation is also known to be involved in the adhesion of leukocytes to artificial biomaterial surfaces (Bruil 1995, Sevast'ianov and Tseytlina 1984, Kazatchkine and Carreno Citation1988). Activation of the complement generates C3a and C5a fragments, which are known to mediate the adhesion and aggregation of leukocytes (Bruil 1995, Kazatchkine and Carreno Citation1988, Hosea et al. Citation1980). Claudia Sperling et al. (Citation2005) have reported that –OH groups materials can activate the complement system significantly compared with –CH3 and –COOH. The C3a and C5a determination results also revealed that PVP-modified PBTNF did not remove the leukocytes by activating the complement.
Conclusions
The hydrophilicity of the PBTNF surface was improved greatly by covalent immobilization of PVP. PVP-modified PBTNFs were investigated for their effect on removing leukocytes and blood compatibility. After filtration, the leukocytes were reduced significantly, while the erythrocytes were reduced slightly. This one-step, convenient modification method can be easily used in applications. In conclusion, the grafting of PVP on the PBTNF could improve its leukocyte-removing efficiency and blood compatibility, suggesting that PVP-modified PBTNF is a very promising blood filter for selective removal of leukocytes.
Declaration of interest
We would like to thank the Sichuan Science and Technology Support Project (project number 2008SZ0101).
References
- Administration TCMAF. 2009. Leukocyte removal filters for single use. Chinese state Food and Drug Administration. Standards Press of China. pp. 14–15.
- Blajchman MA. 1997. Allogeneic blood transfusions, immunomodulation, and postoperative bacterial infection: Do we have the answers yet? Transfusion 37:121 125.
- Brozovic B. 1987. The role of leukocyte depletion in blood transfusion practice. Oxford: Blackwell Scientific Publications.
- Bruil A, Beugeling T, Feijen J, Aken WG. 1995. The mechanisms of leukocyte removal by filtration. Transfusion Medicine Reviews 9: 145 166.
- Bruil A, Brenneisen LM, Terlingen JGA, Beugeling T, Aken WG, Feijen J. 1994. In vitro leukocyte adhesion to modified polyurethane surfaces. II. Effect of wettability. Journal of Colloid and Interface Science 165:72 81.
- Cao Y, Wang H, Yang C, Zhong R, Lei Y, Sun K, Liu J. 2011. In vitro studies of PBT nonwoven fabrics adsorbent for the removal of low density lipoprotein from hyperlipemia plasma. Applied Surface Science 17:7521 7528.
- Cassens U, Greve B, Tapernon K, Nave B, Severin E, Sibrowski W, Gohde W. 2002. A novel true volumetric method for the determination of residual leucocytes in blood components. Vox Sanguinis 82:198 206.
- Dijkstra Tiekstra MJ, Van Der Meer PF, Pietersz RNI, De Wildt Eggen J. 2004. Multicenter evaluation of two flow cytometric methods for counting low levels of white blood cells. Transfusion 44:1319 1324.
- Dzik S, Moroff G, Dumont L. 2000. A multicenter study evaluating three methods for counting residual WBCs in WBC©\reduced blood components: Nageotte hemocytometry, flow cytometry, and microfluorometry. Transfusion 40:513 520.
- Eernisse J, Brand A. 1981. Prevention of platelet refractoriness due to HLA antibodies by administration of leukocyte-poor blood components. Experimental Hematology 9:77.
- El-Rehim H, El-Arnaouty M. 2004. Properties and biocompatibility of polypropylene graft copolymer films. Journal of Biomedical Materials Research Part B: Applied Biomaterials 68:209–215.
- Fang H, Wei J, Yu Y. 2004. In vivo studies of endotoxin removal by lysine-cellulose adsorbents. Biomaterials 25:5433 5440.
- Garvin JE. 1961. Factors affecting the adhesiveness of human leucocytes and platelets in vitro. The Journal of Experimental Medicine 114:51.
- Gerard E, Bessy E, Salvagnini C, Rerat V, Momtaz M, Henard G, Marmey P, Verpoort T, Marchand-Brynaert J. 2011. Surface modifications of polypropylene membranes used for blood filtration. Polymer March 2011; 52(5):1223–1233.
- Heddle NM, Klama L, Meyer R, Walker I, Boshkov L, Roberts R, Chambers S, Podlosky L, O’Hoski P, Levine M. 1999. A randomized controlled trial comparing plasma removal with white cell reduction to prevent reactions to platelets. Transfusion 39:231 238.
- Higuchi A, Shirano K, Harashima M, Yoon BO, Hara M, Hattori M, Imamura K. 2002. Chemically modified polysulfone hollow fibers with vinylpyrrolidone having improved blood compatibility. Biomaterials 23:2659 2666.
- Hirafuji M, Shinoda H. 1991. Platelet-leukocyte interaction in adhesion to endothelial cells induced by platelet-activating factor in vitro. British Journal of Pharmacology 103:1333.
- Hoffman AS. 1982. Blood-biomaterial interactions: An overview. Biomaterials: Interfacial phenomena and applications 199:3C8.
- Hosea S, Brown E, Hammer C, Frank M. 1980. Role of complement activation in a model of adult respiratory distress syndrome. Journal of Clinical Investigation 66:375.
- Kazatchkine M, Carreno M. 1988. Activation of the complement system at the interface between blood and artificial surfaces. Biomaterials 9:30 35.
- Kim EJ, Yeo GD, Pai CM, Kang IK. 2009. Preparation of surface-modified poly (butylene terephthalate) nonwovens and their application as leukocyte removal filters. Journal of Biomedical Materials Research Part B: Applied Biomaterials 90:849–856.
- Lewis A, Freeman R, Redman R, Tolhurst L, Kirkwood L, Grey D, Vick T. 2003. Wettable phosphorylcholine-containing polymers useful in blood filtration. Journal of Materials Science: Materials in Medicine 14:39–45.
- Lewis AL, Hughes PD, Kirkwood LC, Leppard SW, Redman RP, Tolhurst LA, Stratford PW. 2000. Synthesis and characterisation of phosphorylcholine-based polymers useful for coating blood filtration devices. Biomaterials 21:1847 1859.
- Lim F, Cooper SL. 1991. The effect of surface hydrophilicity on biomaterial-leukocyte interactions. ASAIO Journal 37(3):M146.
- Mockel D, Staude E, Guiver MD. 1999. Static protein adsorption, ultrafiltration behavior and cleanability of hydrophilized polysulfone membranes. Journal of Membrane Science 158:63 75.
- Moroff G, Eich J, Dabay M. 1994. Validation of use of the Nageotte hemocytometer to count low levels of white cells in white cell©\reduced platelet components. Transfusion 34:35 38.
- Murphy M, Metcalfe P, Thomas H, Eve J, Ord J, Lister T, Waters A. 1986. Use of leucocyte/poor blood components and HLA/matched/ platelet donors to prevent HLA alloimmunization. British Journal of Haematology 62:529 534.
- Natori SH, Gomei Y, Higuchi A. 2006. Synthesis and performance of amphiphilic copolymers for blood cell separation. Journal of Biomedical Materials Research Part B: Applied Biomaterials 78:318–326.
- Neumann A, Absolom D, Van Oss C, Zingg W. 1979. Surface thermodynamics of leukocyte and platelet adhesion to polymer surfaces. Cell Biochemistry and Biophysics 1:79 92.
- Nishiyama T, Hanaoka K. 2000. Free hemoglobin concentrations in patients receiving massive blood transfusion during emergency surgery for trauma. Canadian Journal of Anesthesia/Journal Canadien d’Anesthesie 47:881–885.
- Rasp F, Clawson C, Repine J. 1981. Platelets increase neutrophil adherence in vitro to nylon fiber. The Journal of Laboratory and Clinical Medicine 97:812.
- Ratner BD. 2004. Biomaterials science: An introduction to materials in medicine. Elsevier (Singapore) Pte Ltd.
- Robinson S, Williams PA. 2002. Inhibition of protein adsorption onto silica by polyvinylpyrrolidone. Langmuir 18:8743 8748.
- Sevast’ianov V, Tseytlina E. 1984. The activation of the complement system by polymer materials and their blood compatibility. Journal of Biomedical Materials Research 18:969 978.
- Sperling C, Schweiss RB, Streller U, Werner C. 2005. In vitro hemocompatibility of self-assembled monolayers displaying various functional groups. Biomaterials 26: 6547 6557.
- Steneker I, Luyn M, Wachem P, Biewenga J. 1992. Electronmicroscopic examination of white cell reduction by four white cell/reduction filters. Transfusion 32:450 457.
- Treleaven JG, McGregor M, Blagdon J. 1984. An evaluation of some of the methods currently available for the production of leucocyte/poor blood. Clinical & Laboratory Haematology 6:45–49.
- Yang C, Cao Y, Sun K, Liu J, Wang H. 2011. Functional groups grafted nonwoven fabrics for blood filtration: The effects of functional groups and wettability on the adhesion of leukocyte and platelet. Applied Surface Science 257:2978 2983.
- Yang C, Sun K, Liu J, Wang H, Cao Y. 2010. Zwitterionic sulfobetaine-modified non-woven fabric for blood filtration. Polymer International 59:1296 1302.



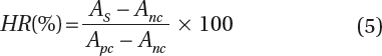
![Figure 4. SEM photos of original PBTNF [erythrocytes (E), leukocytes (L), platelet (P)]; 189 × 274 mm (300 × 300 DPI).](/cms/asset/f0d89430-a630-4e73-b9b0-47aaf96cb491/ianb19_a_657206_f0004_b.gif)