Abstract
This study aims to examine the contribution of PEG-conjugated hemoglobin combined with cisplatin to the expression of HIF-1α and MDR1 in a tumor xenograft model. Cervical carcinoma models were assigned to 4 groups and treated respectively: group 1(control); group 2, cisplatin; group 3, PEG-Hb; group 4 cisplatin plus PEG-Hb. 4 weeks later, tumor volume and MVD was significantly decreased in group 4 compared with other groups. Lower expression of HIF-1α and MDR1 were detected in group4. Taken together, our data indicated that PEG-Hb plus cisplatin can promote tumor tissue oxygenation and enhance the chemotherapy sensitivity. HIF-1α regulated MDR1 pathway correlated with this process.
Introduction
At present, chemotherapy is still one of the major strategies in cancer treatment. But primary or acquired drug resistance is a main obstacle which strictly influences the long-term effect of treatment (Seruga et al. Citation2011, Singh and Settleman Citation2010). Regarding the reasons behind the chemotherapy resistance, more and more attention has been focused on tumor tissue hypoxia, although other factors are also involved into this process. Studies have shown that tumor hypoxia is related to both chemotherapy resistance and radiation resistance (Sullivan et al. Citation2008, Schnitzer et al. Citation2006, Magnon et al. Citation2007). In vivo experiments indicated that when the tumor volume is over 2mm3, tumor growth is angiogenesis-dependent (Norrby Citation2006, Zetter Citation1998). Rapid proliferation of tumor cells and relatively limited, newly formed vascular systems contribute to the hypoxia situation, which accompanies the tumor development.
As an adaptive response, tumor cells have achieved a special ability that enables them to conquer the conditions of hypoxia. For instance, HIF-1α (hypoxia-inducible factor-1 alpha) is easily activated in a hypoxia environment (Pyr dit Ruys et al. Citation2009). Studies proved that HIF-1α is a key transcriptional factor and regulated transcription of a wide range of genes, including angiogenesis (VEGF, TGF) (May et al. Citation2005, Nishi et al. Citation2004), glucose metabolism (AMF/GPI, HK1) (Zhao et al. Citation2007), drug resistance (MDR1) (Ji et al. Citation2010), erythropoiesis (EPO) (Lemus-Varela et al. Citation2010), and cell proliferation (cyclin G2, IGF) (Zhang et al. Citation2011). These adaptive responses can further distort the local microenvironment. As a result, the abnormal, newly formed vascular network and increased MDR1 gene expression inhibit the efficacy of cytotoxic drugs.
The initial purpose for designing artificial oxygen carriers was to substitute blood cells in blood transfusion. Recent research has indicated that a hemoglobin-based oxygen carrier (HBOC) was beneficial in increasing the tissue oxygenation in tumors or ischemia diseases (George et al. Citation2006, Yamamoto et al. Citation2009). Moreover, the recovery of tissue oxygen supply is always accompanied by chemosensitization or radiation sensitization in cancer treatment. Polyethylene glycol-conjugated hemoglobin (PEG-Hb), one type of HBOC, has proved its special advantage in enhancing tumor oxygenation and inhibiting angiogenesis when combined with cisplatin treatment (Han et al. Citation2008, Dai et al. Citation2008). The present study was designed to explore whether MDR1 participated in the chemosensitization process and the relationship of MDR1 and HIF-1α in a cervical carcinoma model treated with PEG-Hb and cisplatin.
Materials and methods
Polyethylene glycol-conjugated hemoglobin (PEG-Hb) used in this study was a Chinese domestic sample. Its design, preparation processes, and physiochemical properties belong to the manufacturer's proprietary and will not be discussed in this paper. The test sample was kept at 4°C in the dark until use.
Cell cultures
The human cervical carcinoma HeLa cell line was obtained from the Cell Biology Center of the Chinese Academy of Medical Sciences (CAMS) and was cultured in RPMI-1640 (Gibco, Life Technologies, Vienna, Australia), supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco), 4mM glutamine,100u/mL penicillin, and 100u/mL streptomycin in 75cm2 tissue plastic flasks (Corning, USA). Cells were incubated at 37 in a humidified 5% CO2 atmosphere.
Establish xenograft tumor model and in vivo experiments
Female SPF BALB/c athymic nude mice, approximately 5wk old, weighing 19 21 g (Institute of Laboratory Animal Sciences, CAMS), were used for this experiment. The animal experiment was approved by the Campus Ethics Committee. After one week of acclimation, 0.2mL (2.5 × 107/mL) HeLa cell suspension was injected subcutaneously into the right flank of nude mice to establish a cervical carcinoma model. Mice were housed at the SPF laboratory and received an aseptic diet and water. Treatment started when tumors reached a volume of 60 80 mm3. Then tumor-bearing animals were randomly assigned to four groups (n = 10) and treated respectively: group 1 (control), physiological saline; group 2, cisplatin (5 mg/kg); group 3, PEG-Hb (0.6 g/kg); group 4 cisplatin (5 mg/kg) plus PEG-Hb (0.6 g/kg). The drugs and saline control were injected via tail vein, twice a week, for four weeks. In this study, investigators performing the following detections were blinded to the mice group setting detail. Twice a week the volume of the transplanted tumor was calculated as (length × width2)/2. 4 weeks later, mice were sacrificed, and isolated tumors were weighed. Treatment efficacy was represented by percent tumor growth inhibition (TGI). The details of the above measurements have been introduced in a past study.
Immunohistochemistry
On the last day of therapy, mice were sacrificed and tumors removed. They were then fixed in 10% formalin and embedded in paraffin. Tumor immunohistochemical (IHC) analysis was performed according to the standard protocols. Sections were incubated with each primary antibody (HIF-1α, CD31, and MDR1, Santa Cruz, USA) at the reference working concentration overnight at 4°C. After being washed three times with PBS, sections were incubated with biotinylated secondary antibodies (1: 50) (Dako, Copenhagen, Denmark). HRP/DAB Kit (Dako, Copenhagen, Denmark) was used under the manufacturer's instructions. The degree of staining was detected with the average optical density of positive signal using Image Pro Plus 4.0 software.
Western blot
For HIF-1α and MDR1 protein expression analysis, fresh isolated tumors were ground in liquid nitrogen and lysed with a cold RIPA protein lysis buffer on ice. The lysates were transferred to Eppendorf tubes and centrifuged at 12,000 g for 10 minutes at 4°C. The supernatants were stored and the total protein concentrations were detected with the Bradford method (Bio-Rad Laboratories, USA). 50 μl total protein was loaded per lane and segregated by SDS-PAGE (5% stacking gel and 8% separating gel), then the separated proteins were transferred electrophoretically from gel to PVDF membrane (Millipore Corp, USA). The membrane incubated with each of the primary antibodies HIF-1α (120KD), MDR1 (170KD), and control antibody β-actin (43KD) (Santa Cruz, USA), respectively, overnight at 4°C with 1:500 dilution. The membrane was incubated for another 1h with horseradish peroxidase-conjugated anti-rabbit or anti-goat IgG (Santa Cruz, USA). Protein bands were revealed using an enhanced chemiluminescence (ECL) kit (Santa Cruz Biotechnology), and then quantified and analyzed with Bandscan software.
Statistic analysis
All statistical calculations were conducted in SPSS 13.0 for Windows and differences were analyzed with two-tail student t test. Pearson correlation coefficients were determined for HIF-1α and MDR1 using data from a Western blot analysis. All data are presented as mean ± SEM. A value of P < 0.05 was considered as statistically significant.
Results
Tumor growth and therapy efficacy in mice
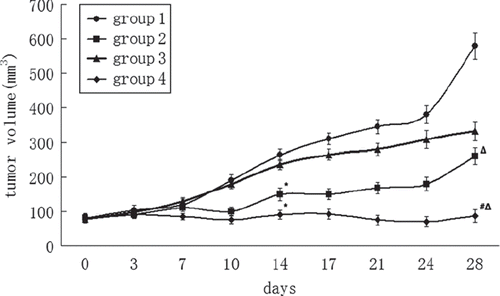
Tumor volumes were recorded in a four-week period. In group 1 (control) and group 3 (PEG-Hb) the tumors developed quickly and constantly. Tumor mass had a little decrease in group 3 (PEG-Hb) on the last day of therapy, but the difference was not significant compared with the control group. In group 2 (cisplatin), the first two weeks’ tumor growth was better controlled because of the anticancer effect of cisplatin, but four weeks later the tumor volume became larger again. In group 4 (cisplatin plus PEG-Hb), tumor volume was significantly inhibited compared with other groups (P < 0.01) (). When treated, the mice with cisplatin combined with PEG-Hb in group 4, and the tumor growth inhibition (TGI) was raised from 74% to 81%, indicating the chemosensitivity effect of PEG-Hb.
Expression of CD31, HIF-1α, and MDR1 in tumor tissue
Microvessel density (MVD) was qualified by light microscopic analysis in the regions that had the most microvessels by two investigators independently. In the present study, the tumor MVD was assessed using the immunostaining of CD31. CD31 also refers to PECAM-1, a glycoprotein expressed on the cell surfaces of monocytes, neutrophils, platelets, and a subpopulation of T cells. Generally, it is used to select and identify vascular endothelial cells. According to Weidner's method (Citation1995), MVD in the tumor tissues was quantified as follows: group 1 (35.2 ± 14.7), group 2 (26.5 ± 13.2), group 3 (28.2 ± 17.5), group 4 (21.9 ± 13.6). Data analysis indicated that MVD was deeply decreased in the PEG-Hb combined cisplatin group 4 compared with the other three groups (P < 0.05). It should be noted that, compared with the control group, MVD was only a little reduced in group 3 (PEG-Hb) and the difference was not significant.
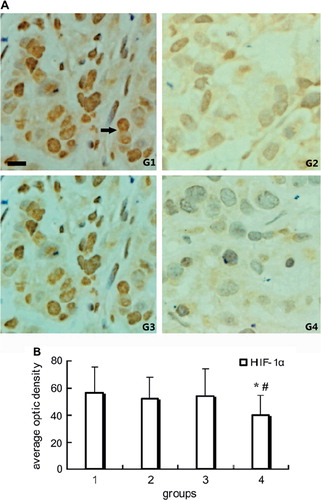
To access the influence of PEG-Hb on the tumor hypoxia, HIF-1α, the important gene transcription factor in adaptive processes to hypoxia, was detected in the current study. The immonohistochemical results showed that the higher HIF-1α expression regions was far from the microvessels, and its expression was obviously inhibited in group 4 compared with other groups (P < 0.05). The representative images and positive staining quantitative analysis were shown in .
Figure 2. Immunohistochemical staining of HIF-1α in tumor tissue: A. Representative pictures of immunohistochemical staining of HIF-1α in groups. Intense cytoplasmic and nucleus staining of HIF-1α in control. Positive stain of HIF-1α (black arrow). B. Quantitative analysis of average optic density of positive stain in specimen. Note *P < 0.01 compared with control; #P < 0.05 compared with group 2. Scale bar, 25μm for G1-G4.
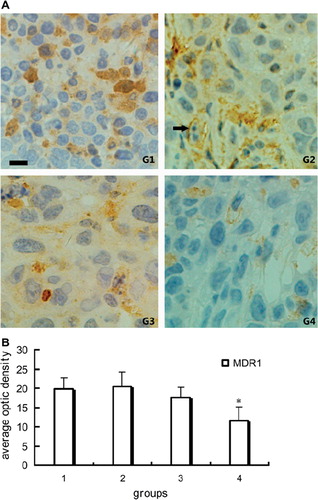
MDR1 is a membrane glycoprotein which acts as a drug efflux transporter, controlling the intercellular drug concentration and is responsible for the multidrug resistance. It was clearly shown that the expression of MDR1 decreased significantly in group 4. Administration of PEG-Hb alone (group 3) could not reduce MDR1 expression obviously ().
Figure 3. Immunohistochemical staining of MDR1 in tumor tissue: A. Representative pictures of immunohistochemical staining of MDR1 in groups. Positive stain of MDR1 (black arrow). B. Quantitative analysis of average optic density of positive stain in specimen. *P < 0.01 compared with other three groups; scale bar, 25 μm for G1-G4.
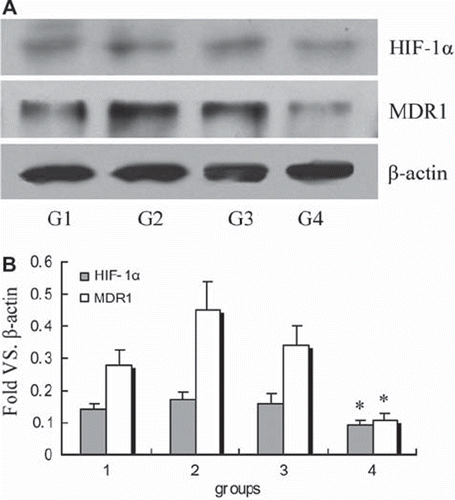
Western blot analysis using anti-HIF-1α and anti-MDR1 antibodies, respectively, and the results showed that, except for group 4, the other three groups express two prominent and specific immunoreactive bands at Mr ∼120,000 and ∼170,000, corresponding to the HIF-1α and MDR1 proteins (). These findings demonstrated that PEG-Hb combined cisplatin administration contributed to the decreased expression of HIF-1α and MDR1. Comparing the expression pattern of HIF-1α to that of MDR1 presented high correlation values (r = 0.87, P < 0.01).
Discussion
Resistance to chemotherapy and radiotherapy is still the main reasons for cancer therapeutic failure even though new anti-cancer drugs have been developed every year. Studies have revealed that the drug resistance is always related to some ATP-binding cassette (ABC) multidrug transporters in tumor cells (Ohishi et al. Citation2002). MDR1 is a typical ABC multidrug transporter, as a membrane glycoprotein drug efflux transporter, controlling the intercellular drug concentration and is responsible for multidrug resistance. More evidence have proved that over-expression of MDR1 is an adverse prognostic factor in cancers (Tanaka et al. Citation2011). In analyzing MDR1 gene sequence, a binding site for hypoxia inducible factor-1 (HIF-1) has been identified, and further functional detection has indicated that inhibition of HIF-1 expression could induce a nearly complete loss of basal MDR1 expression (Comerford et al. Citation2002).
Tumor hypoxia is not only related to tumor development and progression but also to chemotherapy and radiotherapy response. Hypoxia is a common feature of many malignant tumors; HIF-1 is the key regulator in altering and adapting the biological characteristic of tumor hypoxia environment. The biological adaptive responses include angiogenesis, glucose metabolism, drug resistance, and cell proliferation. These adaptive responses can further distort the local microenvironment. So, in targeting tumor hypoxia, a variety of strategies has been designed to improve tumor oxygenation, therefore reversing multidrug and radiation resistance. These efforts, such as hyperoxic gas inhalation (Kaanders et al. Citation2004), VEGF blockade (Ansiaux Citation2005), and artificial oxygen carriers. Artificial oxygen carriers are beneficial in overcoming tumor hypoxia by improving oxygen supply. Several studies have shown promising light in combination with conventional cancer treatment, such as hemoglobin vesicles in cancer irradiation (Yamamoto et al. Citation2009), ultrapurified polymerized bovine hemoglobin solution in gliosarcoma irradiation (Teicher et al. Citation1994, Robinson et al. Citation1995), and PEG-conjugated bovine hemoglobin in radiotherapy in rodent models (Nozue et al. Citation1996). In our previous studies, we have observed that cisplatin combined with PEG-Hb (0.6 g/kg) was beneficial to tumor tissue oxygenation, therefore decreasing the tumor angiogenesis and down-regulation of VEGF (Han et al. Citation2008, Dai et al. Citation2008, Yu et al. Citation2008).
In our present study, when cisplatin administration combined with PEG-Hb in group 4, the tumor growth inhibition (TGI) was raised from 74% to 81%. To reveal the possible patterns contributing to PEG-Hb chemosensitivity, we investigated the expression of MDR1 in tumor tissue. We found that decreased MDR1 as well as HIF-1α downregulation and MVD reduction effects in cervical carcinoma xenograft with co-administration of cisplatin and PEG-Hb (group 4) were more pronounced that that produced by cisplatin alone (group 2). In addition, when comparing the expression pattern of HIF-1α to that of MDR1, it presented high correlation values.
We speculated that downregulation of MDR1 might be associated with decreased HIF-1α protein levels when cisplatin plus PEG-Hb was administered. Experiments have proved that HIF-1α and MDR1 have a close relationship. Expression of HIF-1α and MDR1 can be detected in part of the sacral chordoma and chordoma cell line CM-319. An immunoreactivity score of HIF-1a was higher in cases showing MDR1 (Ji et al. Citation2010). Ding's data (Citation2010) indicated that the expression of HIF-1α in colon carcinoma hypoxia environment may be associated with the gene MDR1 and interactively participated in the tumor multidrug resistance. Han et al. (Citation2007) designed an experiment using HIF-1α siRNA, which can completely inhabit the expression of MDR1 in H4IIE rat hepatoma cells. The above data generally indicate that hypoxia induces expression of HIF-1α and MDR1/P-gp in some tumor tissues or cancer cell lines. However, it is currently unclear how HIF-1α may regulate the expression of MDR1.
In conclusion, the results of this study suggest that PEG-Hb plus cisplatin can promote tumor tissue oxygenation and enhance the chemotherapy sensitivity. The HIF-1α regulated MDR1 pathway may associate with PEG-Hb chemosensitization. However, it should be noted that HIF-1α regulates variety genes which participated in tumor hypoxia adaption. Other possible HIF-1α regulated factors that contributed to PEG-Hb chemosensitization need to be further investigated.
Acknowledgment
This work was supported by Prof. Ruijuan Xiu's UNESCO Award for Woman in Science 2000 and the grant of “Knowledge Innovation Project” Academy of Science, China (No. KJCX1-SW-07).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ansiaux R, Baudelet C, Jordan BF, Beghein N, Sonveaux P, De Wever J, Martinive P, Gregoire V, Feron O, Gallez B. 2005. Thalidomide radiosensitizes tumors through early changes in the tumor microenvironment. Clin Cancer Res. 11(2):743–750.
- Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. 2002. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 62(12):3387–3394.
- Dai M, Yu M, Han J, Li H, Cui P, Liu Q, Xiu R. 2008. PEG-conjugated hemoglobin combination with cisplatin enforced the antiangiogeic effect in a cervical tumor xenograft model. Artif Cells Blood Substit Immobil Biotechnol. 36(6):487–497.
- Ding Z, Yang L, Xie X, Xie F, Pan F, Li J, He J, Liang H. 2010. Expression and significance of hypoxia-inducible factor-1 alpha and MDR1/P-glycoprotein in human colon carcinoma tissue and cells. J Cancer Res Clin Oncol. 136(11):1697–707.
- George I, Yi GH, Schulman AR, Morrow BT, Cheng Y, Gu A, Zhang G, Oz MC, Burkhoff D, Wang J. 2006. A polymerized bovine hemoglobin oxygen carrier preserves regional myocardial function and reduces infarct size after acute myocardial ischemia. Am J Physiol Heart Circ Physiol. 291(3):H1126–H1137.
- Han HK, Han CY, Cheon EP, Lee J, Kang KW. 2007. Role of hypoxia-inducible factor-alpha in hepatitis-B-virus X protein-mediated MDR1 activation. Biochem Biophys Res Commun. 357(2):567–573.
- Han J, Yu M, Dai M, Cui P, Li H, Zhang J, Liu Q, Xiu R. 2008. Effect of artificial oxygen carrier with chemotherapy on tumor hypoxia and neovascularization. Artif Cells Blood Substit Immobil Biotechnol. 36(5):431–438.
- Ji Z, Long H, Hu Y, Qiu X, Chen X, Li Z, Fan D, Ma B, Fan Q. 2010. Expression of MDR1, HIF-1alpha and MRP1 in sacral chordoma and chordoma cell line CM-319. J Exp Clin Cancer Res. 29:158.
- Kaanders JH, Bussink J, van der Kogel AJ. 2004. Clinical studies of hypoxia modification in radiotherapy. Semin Radiat Oncol. 14(3):233–240.
- Lemus-Varela ML, Flores-Soto ME, Cervantes-Munguia R, Torres-Mendoza BM, Gudino-Cabrera G, Chaparro-Huerta V, Ortuno-Sahagun D, Beas-Zarate C. 2010. Expression of HIF-1 alpha, VEGF and EPO in peripheral blood from patients with two cardiac abnormalities associated with hypoxia. Clin Biochem. 43(3):234–239.
- Magnon C, Opolon P, Ricard M, Connault E, Ardouin P, Galaup A, Metivier D, Bidart JM, Germain S, Perricaudet M, Schlumberger M. 2007. Radiation and inhibition of angiogenesis by canstatin synergize to induce HIF-1alpha-mediated tumor apoptotic switch. J Clin Invest. 117(7):1844–1855.
- May D, Itin A, Gal O, Kalinski H, Feinstein E, Keshet E. 2005. Ero1-L alpha plays a key role in a HIF-1-mediated pathway to improve disulfide bond formation and VEGF secretion under hypoxia: implication for cancer. Oncogene. 24(6):1011–1020.
- Nishi H, Nakada T, Hokamura M, Osakabe Y, Itokazu O, Huang LE, Isaka K. 2004. Hypoxia-inducible factor-1 transactivates transforming growth factor-beta3 in trophoblast. Endocrinology. 145(9):4113–4118.
- Norrby K. 2006. In vivo models of angiogenesis. J Cell Mol Med. 10(3):588–612.
- Nozue M, Lee I, Manning JM, Manning LR, Jain RK. 1996. Oxygenation in tumors by modified hemoglobins. J Surg Oncol. 62(2):109–114.
- Ohishi Y, Oda Y, Uchiumi T, Kobayashi H, Hirakawa T, Miyamoto S, Kinukawa N, Nakano H, Kuwano M, Tsuneyoshi M. 2002. ATP-binding cassette superfamily transporter gene expression in human primary ovarian carcinoma. Clin Cancer Res. 8(12):3767–3775.
- Pyr dit Ruys S, Delaive E, Demazy C, Dieu M, Raes M, Michiels C. 2009. Identification of DH IC-2 as a HIF-1 independent protein involved in the adaptive response to hypoxia in tumor cells: A putative role in metastasis. Biochim Biophys Acta. 1793(11):1676–1690.
- Robinson MF, Dupuis NP, Kusumoto T, Liu F, Menon K, Teicher BA. 1995. Increased tumor oxygenation and radiation sensitivity in two rat tumors by a hemoglobin-based, oxygen-carrying preparation. Artif Cells Blood Substit Immobil Biotechnol. 23(3):431–438.
- Schnitzer SE, Schmid T, Zhou J, Brune B. 2006. Hypoxia and HIF-1alpha protect A549 cells from drug-induced apoptosis. Cell Death Differ. 13(9):1611–1613.
- Seruga B, Ocana A, Tannock IF. 2011. Drug resistance in metastatic castration-resistant prostate cancer. Nat Rev Clin Oncol. 8(1):12–23.
- Singh A, Settleman J. 2010. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene. 29(34):4741–4751.
- Sullivan R, Pare GC, Frederiksen LJ, Semenza GL, Graham CH. 2008. Hypoxia-induced resistance to anticancer drugs is associated with decreased senescence and requires hypoxia-inducible factor-1 activity. Mol Cancer Ther. 7(7):1961–1973.
- Tanaka M, Okazaki T, Suzuki H, Abbruzzese JL, Li D. 2011. Association of multi-drug resistance gene polymorphisms with pancreatic cancer outcome. Cancer. 117(4):744–751.
- Teicher BA, Holden SA, Dupuis NP, Kusomoto T, Liu M, Liu F, Menon K. 1994. Oxygenation of the rat 9L gliosarcoma and the rat 13672 mammary carcinoma with various doses of a hemoglobin solution. Artif Cells Blood Substit Immobil Biotechnol. 22(3):827–833.
- Weidner N. 1995. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 36(2):169–180.
- Yamamoto M, Izumi Y, Horinouchi H, Teramura Y, Sakai H, Kohno M, Watanabe M, Kawamura M, Adachi T, Ikeda E, Takeoka S, Tsuchida E, Kobayashi K. 2009. Systemic administration of hemoglobin vesicle elevates tumor tissue oxygen tension and modifies tumor response to irradiation. J Surg Res. 151(1):48–54.
- Yu M, Han J, Dai M, Cui P, Li H, Liu Q, Xiu R. 2008. Influence of PEG-conjugated hemoglobin on tumor oxygenation and response to chemotherapy. Artif Cells Blood Substit Immobil Biotechnol. 36(6):551–561.
- Zetter BR. 1998. Angiogenesis and tumor metastasis. Annu Rev Med. 49:407–424.
- Zhang C, Lu L, Li Y, Wang X, Zhou J, Liu Y, Fu P, Gallicchio MA, Bach LA, Duan C. 2011. IGF binding protein-6 expression in vascular endothelial cells is induced by hypoxia and plays a negative role in tumor angiogenesis. Int J Cancer. available online. doi: 10.1002/ijc.26201.
- Zhao Q, Du J, Gu H, Teng X, Zhang Q, Qin H, Liu N. 2007. Effects of YC-1 on hypoxia-inducible factor 1-driven transcription activity, cell proliferative vitality, and apoptosis in hypoxic human pancreatic cancer cells. Pancreas. 34(2):242–247.