Abstract
The water-soluble poly(methyl vinyl ether-co-maleic anhydride) copolymer-bovine serum albumin bioconjugates were synthesized in the presence of 1-ethyl-3-(3-dimetilamino-propyl) carbodiimide hydrochloride as cross-linking agents via microwave-assisted and conventional methods and characterized by size-exclusion chromatography and high-performance liquid chromatography. According to size-exclusion chromatography and high-performance liquid chromatography results, the bioconjugates synthesized in the microwave-assisted method are more stable and efficient than the conventional method. The reaction time is shortened from 17 hours to 15 minutes by means of the microwave-assisted method.
| Abbreviations | ||
| P(MVE-MA) | = | Poly (methyl vinyl ether-co-maleic anhydride) copolymer |
| BSA | = | Bovine serum albumin |
| WSPE | = | Water-soluble polyelectrolyte |
| MA | = | Microbial antigen |
| MWC | = | Microwave-assisted conjugates |
| CMC | = | Conventional method conjugates |
| MWM | = | Microwave-assisted method |
| CM | = | Conventional method |
| EDC | = | 1-ethyl-3-(3-dimetilaminopropyl) karbodiimid hydrochloride |
| SEC | = | Size-exclusion chromatography |
| HPLC | = | High-performance liquid chromatography |
Introduction
Water-soluble polyelectrolyte (WSPE)-microbial antigen (MA) (proteins, peptide, polysaccharides, etc.) conjugates have been the subject of many studies in the application areas of biotechnology, medicine, and pharmacy. The WSPEs are not immunogenic, possess carrier properties and adjuvant activities simultaneously, and provide effective immuno response. The use of this WSPE as the carriers of MA has made it possible not only to increase the immune responsiveness of the organism by several orders of magnitude, but it also provides effective immune protection (Mustafaev Citation1996, Karahan et al. Citation2010, Mansuroğlu et al. Citation2011, Karahan Citation2009, Petrov et al. Citation1992, Mustafaev and Sarac Citation1996, Dilgimen et al. Citation2001, Mustafaev and Norimov Citation1990, Mustafaev et al. Citation1990, Mustafaev et al. Citation1996, Dincer et al. Citation1997, Shoukry et al. Citation2002, Akkiliç et al. Citation2007, Wu Citation2004, Karahan et al. Citation2007, Guney et al. Citation1997, Peters Citation1985, Peters Citation1996, Mustafaev Citation2004, D’Souza et al. Citation2004, cooper et al. Citation2005, Topuzogullari et al. Citation2007).
Maleic anhydride copolymers, which become polyelectrolytes in an aqueous medium due to the hydrolysis of anhydride to the ring, are known to be effective in biomedical applications of drug transport systems and enzyme immobilization (Ladaviere et al. Citation1999). Several characteristic properties have been studied; namely, the two-step dissociation of carboxylic groups (Minakata et al. Citation1980) and binding of counterions (Kitano et al. Citation1987), pH-induced conformation transitions (Kawaguchi et al. Citation1991), and a remarkable behavior of viscosity that exhibits a maximum at the half-neutralization point (Minakata et al. Citation1980, Kitano et al. Citation1987, Kawaguchi et al. Citation1991, Ohno and Sugai Citation1990). Also, these copolymers proved, so far, the most capable at binding molecules as different as nucleic acids and proteins such as BSA, which is used as a model protein in many studies (Ladaviere et al. Citation1997, Wang et al. Citation2008).
WSPE-MAs were synthesized via conventional methods such as complexes by physical mixture, complexes in the presence of metal, conjugates via spacers or varied cross-linkers, etc. However, conventional methods require a long reaction time (Mustafaev Citation1996, Karahan et al. Citation2010, Mansuroğlu et al. Citation2011, Karahan Citation2009, Dilgimen et al. Citation2001, Mustafaev and Norimov Citation1990, Mustafaev et al. Citation1990, Akkiliç et al. Citation2007, Karahan et al. Citation2007). For this purpose, we aimed to show shorter reaction time using microwaves to synthesize WSPE-MA conjugates. The microwave-assisted method involves the use of microwave radiations as an impact on chemical synthesis. Microwave-assisted reactions are provided by the thermal and/or non-thermal effect of this radiation energy. Microwave energy is considered to be due to dipole interactions providing a vibration feature to the molecules so that they have a significant impact for the conjugation reaction. This feature is called a non-thermal feature of microwave energy (non-thermal effects) (Budarin et al. Citation2011, Alesi et al. Citation2008).
In this study, to develop the synthetic polymeric vaccine model systems, the poly(methyl vinyl ether- co-maleic anhydride) copolymer-bovine serum albumin (BSA) bioconjogates which were obtained by using 1-ethyl-3-(3-dimetilaminopropyl) carbodiimide hydrochloride (EDC) by conventional and microwave-assisted methods were performed in varying rations at pH 7.0. All of the bioconjugates were characterized by size-exclusion chromatography and high-performance liquid chromatography methods.
Methods
Materials
Poly(methyl vinyl ether-co-maleic anhydride) copolymers (molecular weight: 41 kDa, Gantiez AN129BF) were supplied from ICP Europe and used without further treatment. The molecular weight of P(MVE-MA) (pK: 3.64 kJ.mol−1) (Jens et al. Citation2007) in phosphate buffer solution at pH 7.0 was found to be Mw: 70 kDa, Mn: 63.388 kDa, Mz: 78.934 kDa by the measurement technique of size exclusion chromatography. Bovine serum albumin (Mw: 66 kDa, pI: 4.9) (Neurath and Bailey Citation1953), 1-ethyl-3-(3-dimetilaminopropyl) carbodiimide hydrochloride (EDC), and dimethyl sulfoxide (DMSO) were purchased from Sigma Chemical Company (St. Louis, USA); other chemicals such as NaOH (Fluka), sodium dihydrogen phosphate (NaH2PO4, Reiadel de Haën), disodyum hydrogen phosphate (Na2HPO4, Fluka), and NaN3 (Applichem) were used without further treatment. Ultra-pure water was obtained from the Millipare Milli-Q gradient system. The solutions of P(MVE-MA) used in this study were prepared in DMSO solvent at room temperature with stirring over 12 h. The solutions of BSA were prepared in phosphate-buffered saline PBS) at pH 7.0.
HPLC gel filtration
BSA, polyelectrolyte (PE), and water-soluble PE-BSA bioconjugates were separated using HPLC. The molecular masses of proteins and the fraction compositions of the polymer–protein mixtures or conjugates were estimated by gel filtration chromatography, using column Shim-Pack Diol-300 (7.9 mm ID×50 cm) with Shim-Pack Pre-column Diol (4.0 mm ID×5 cm) at room temperature. A Shimadzu model LC-6AD pump was run in different buffers at a flow rate of 1.0 ml/min. All solutions were filtered with 0.45 μm Sartorius RC-membrane filters before injection. A 20 μl sample volume was injected for analysis.
The eluate was monitored at 280 nm with Shimadzu SPD-10AV VP Model UV–vis Detector. PE-BSA bioconjugates were fractioned using a Shimadzu FRC-10A model fraction collector. The standards used to calibrate the column were thyroglobulin (670 kDa), immunoglobulin (155 kDa), BSA (66 kDa), ovalbumin (44 kDa), and myoglobin (16.9 kDa).
PBS was prepared from the Millipore MilliQ Gradient system, and consisted of 50 mM phosphate and 150 mM sodium chloride for pH 7.0 studies. Mobile phase solutions were filtered through a 0.45 μm cellulose nitrate filter and were degassed before use.
SEC measurements
A Viscotek TDA 302 detector system with refractive index (660 nm), right angle light scattering (670 nm), and four-capillary differential viscometer detectors was used for on-line SEC signal detection. A separate UV detector obtained from Viscotek was connected to this detector system and the detectors were in the following order: UV–LS–RI–VIS. 0.2 μm nylon pre-filter that was used between the column and detectors; HPLC pump, degasser, and autosampler with 100 μl injection loop with built-in Viscotek GPCmax VE 2001 pump system, which is connected to the detectors. Omni SEC 4.1 software was used for the acquisition and analysis of SEC data.
A Viscotek quadruple detector array was calibrated using a BSA monomer peak in a mobile phase of phosphate- buffered saline (PBS) at 1.0 ml/min flow rate 0.185 (Kendrick et al. Citation2001) and 0.66 (Wen et al. Citation1996) were used as dn/dc value and extinction coefficient of BSA, respectively. A Shim-Pack Diol 300 column in the dimensions of 500 mm length and 7.9 mm inlet diameter was used for separating BSA, PE, and PE-BSA conjugates. Elution was isocratic and at a flow rate of 1.0 ml/min. PBS was prepared using ultra-pure water from a Millipore MilliQ Gradient system, and consisted of 50 mM phosphate and 150 mM sodium chloride; pH 6.0 and 7.0. buffer solutions were filtered through a 0.45 μm Millipore cellulose nitrate filter and were degassed before use.
Microwave (multi-mode)
Milestone's MicroSYNTH (Sorisole, Italy) labstation, combined with the microwave technology, satisfies the needs of the various chemical research laboratories. In fact, the different accessories that can be fitted inside the multimode microwave cavity cover a large ramp of volume, from 2 ml to 500 ml, different values of pressure from 1 to 50 bar, and give the possibility of reaching temperatures up to 250oC. Researchers can also choose to perform one reaction at a time or to perform parallel synthesis with Milestone's rotors for up to 24 reactions at the same time (Favretto Citation2003).
Synthesis of WSPE-BSA bioconjugates
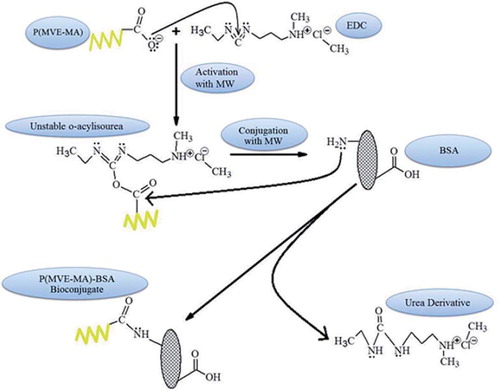
Cross-linking agents (cross-binders) are chemical reagents that ensure the formation of covalent bonds of molecules and have many different varieties, according to the application purposes in processes. They are known as EDC or EDAC 1-ethyl-3-(3-dimetilaminopropil) carbodiimide hydrochloride derivatives of the most commonly used conjugation reaction of biological molecules. Conjugation of the molecule that contains primary amine and carboxyl groups can be realized using EDC. According to the conjugate formation process, primarily the N-substituted carbodiimides react with carboxylic acids and they form the highly reactive O-acylisourea intermediate product. Then, the active intermediate product reacts with nucleophilic groups such as the primary amines to perform the conjugates via amide bond formation. According to the literature, to ensure the highest activity of carbodiimides the optimum pH range is 4.7 to 6.0 (Hermanson Citation1996).
P(MVE-MA)-BSA bioconjugates were synthesized via two methods: conventional and microwave-assisted. A schematic representation of the reaction procedure is shown in .
In order to produce P(MVE-MA)-BSA bioconjugates with a conventional method, the solution of poly(methyl vinyl ether-co-maleic anhydride) (1 mg) dissolved in DMSO (0.1 ml), stirred at room temperature and 1.9 ml PBS was added to the solution to ensure the amount of organic phase was at a maximum 5% rate of total solvent, but to prevent any possible formation of micelles, PBS was added under rapid mixing. EDC was added more than four times the amount of the copolymer (4 mg), and pH of solution was adjusted to 4.7 in order to provide the highest reactivity of the EDC. After 3 h stirring, BSA solution in different concentrations (nBSA/nP(MVE-MA): 0.25, 0.5, 1.0, 3.0 and 5.0) () was added to the reaction mixture and stirred for 12 h. The total volume of the obtained solution was completed to 4 ml and was stirred 2 h more than the pH of solution, which was adjusted to 7.0 using 1 N NaOH solution prepared in ultra-pure water. The total reaction time was 17 h.
Table I. The experimental ratios and concentrations of nBSA/nP(MVE-MA) [BSA Mw:66 kDa and P(MVE-MA) Mw:70 kDa].
In order to obtain P(MVE-MA)-BSA bioconjugates via the microwave-assisted method, the solution of poly(methyl vinyl ether-co-maleic anhydride) (1 mg) was dissolved in 0.1 ml DMSO, stirred at room temperature, 1.9 ml PBS was added to the solution, and EDC was added in more than four times the amount of the copolymer (pH 4.7). After 3 min stirring of solution under microwave [75 Watt (W), Microsynth, Milestone®, BG, Italy], to compare the results with the classical method, BSA solution in different concentrations (nBSA/nP(MVE-MA): 0.25, 0.5, 1.0, 3.0 and 5.0) () was added to the reaction mixture and stirred for (75 W) 10 min. The total volume of the obtained solution was completed to 4 ml and was stirred (75 W) 2 min more, then the pH value of the mixture was adjusted to 7.0 with 1N NaOH. The total reaction time was 15 min. The BSA/poly(methyl vinyl ether-co-maleic anhydride) copolymers ratios, nBSA/nP(MVE-MA), were calculated using the equation n = CNA/M, where n is the number of the molecules in 1 ml, M is the molecular weight of components, NA is the avagadro's number, and C represents concentration in g/ml. Results are given in .
Results and discussion
To compare the CM and MWM by means of reaction time, the reaction time was shortened from 17 h to 15 min via MWM.
HPLC and SEC were used for the P(MVE-MA)-BSA bioconjugates formation. HPLC chromatograms belonging to CMC and MWC are given in and , respectively. According to these chromatograms, the trimer peak of BSA proteins was seen between 13.5 and 19 min. P(MVE-MA) copolymer didn't show any peak-like baseline, but as a result of the BSA and P(MVE-MA) interactions, the peaks of the conjugates have been observed between 9 and 11.5 minutes because of the occurrence of BSA-copolymer conjugates, which have larger molecular size and molecular weight than free-form P(MVE-MA). When obtained conjugates are compared among themselves, if the added amount of BSA to a fixed amount of polymer rises, intensity increases of the conjugate peaks are observed. When comparing the CM and MWM, larger peak areas were observed in MWM due to the bigger molecular size and weight of P(MVE-MA)-BSA bioconjugates than CM.
Figure 1. Conventional Method Conjugates (CMC): HPLC chromatograms of BSA, P(MVE-MA), and BSA-P(MVE-MA) conjugates prepared at ratio on nBSA/nP(MVE-MA): 0.25(1), 0.5(2), 1(3), 3(4), 5(5).
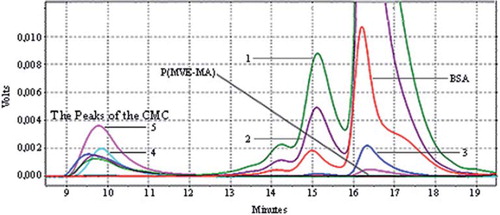
Figure 2. Microwave Assisted Conjugates (MWC): HPLC chromatograms of BSA, P(MVE-MA), and BSA-P(MVE-MA) conjugates prepared at ratio on nBSA/nP(MVE-MA): 0.25(1), 0.5(2), 1(3), 3(4), 5(5).
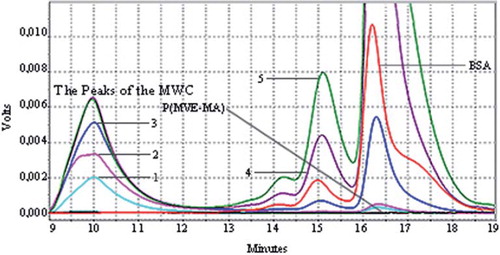
The UV and LS chromatograms of synthesized P(MVE-MA)-BSA bioconjugates using CM and MWM are illustrated in , and ,, respectively. These figures show the chromatograms of P(MVE-MA)-BSA bioconjugates at different ratios of molecular concentrations of components (nBSA/nP(MVE-MA)) at pH 7.0, and it can be seen that, with an increase in the number of BSA molecules in the bioconjugate [the concentration of P(MVE-MA) is kept constant], the areas of bioconjugates’ chromatographic peaks increase, as were obtained by UV and LS detectors (Topuzogullari et al. Citation2007). According to these figures, the highest peak intensities were obtained in nBSA/nP(MVE-MA): 5.
Figure 3. SEC [UV (a), LS (b)] chromatograms of the conjugates by conventional method of BSA-P(MVE-MA) bioconjugates prepared at the ratios of nBSA/nP(MVE-MA): 0.25(1), 0.5(2), 1(3), 3(4), 5(5).
![Figure 3. SEC [UV (a), LS (b)] chromatograms of the conjugates by conventional method of BSA-P(MVE-MA) bioconjugates prepared at the ratios of nBSA/nP(MVE-MA): 0.25(1), 0.5(2), 1(3), 3(4), 5(5).](/cms/asset/1a9cf134-a7e7-47dc-a951-534ff707990e/ianb19_a_678942_f0003_b.jpg)
Figure 4. SEC [UV (a), LS (b)] chromatograms of the conjugates by means of the microwave-assisted method of BSA-P(MVE-MA) bioconjugates prepared at the ratios of nBSA/nP(MVE-MA): 0.25(1), 0.5(2), 1(3), 3(4), 5(5).
![Figure 4. SEC [UV (a), LS (b)] chromatograms of the conjugates by means of the microwave-assisted method of BSA-P(MVE-MA) bioconjugates prepared at the ratios of nBSA/nP(MVE-MA): 0.25(1), 0.5(2), 1(3), 3(4), 5(5).](/cms/asset/ba4587f6-9f98-4320-9cef-4d6abe4eac7e/ianb19_a_678942_f0004_b.jpg)
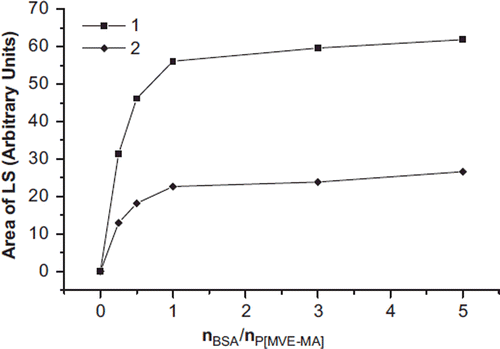
The area of UV and LS of bioconjugates is plotted in and . UV areas MWC were four times bigger than CMC, and LS areas of MWC were three times larger than CMC. That is, in direct proportion to the CMC and MWC, the peak area seems to have increased when nBSA/nP(MVE-MA) ratio increased. The molecular weights of the CMC and MWC increase depending on molecule sizes.
Figure 5. The area of the UV of the conjugates (MWC) (1) and conjugates (CMC) (2) prepared at the ratios of nBSA/nP(MVE-MA): 0.25, 0.5, 1, 3, 5.
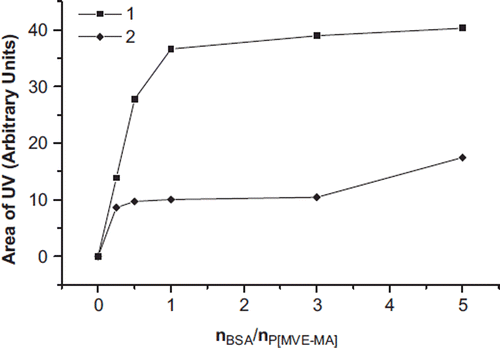
Figure 6. The area of the LS of the conjugates (MWC) (1) and conjugates (CMC) (2) prepared at the ratios of nBSA/nP(MVE-MA): 0.25, 0.5, 1, 3, 5.
As a result of the measurements, as long as the greater amount of BSA was banded to P(MVE-MA), the larger peak was observed. In addition, the reaction time of P(MVE-MA)-BSA bioconjugates under microwave-assisted conditions was shortened from 17 h to 15 min because of the non-thermal effect of the microwave energy's dipolar polarization mechanism on the molecules.
Conclusion
Water-soluble and stable P(MVE-MA)-BSA bioconjugates have been synthesized successfully via a microwave-assisted method with shortened reaction time. The best ratio of bioconjugates was determined to be nBSA/nP(MVE-MA): 5. It is suggested that microwave-assistance is a useful technique to develop a synthetic vaccine model system by means of short reaction time and synthesized stable bioconjugates without protein denaturation.
Acknowledgements
The authors dedicate this report to the memory of the Founder Head of Y ld z Technical University Bioengineering Department, the precious man of science, Prof. Dr. M.I. Mustafaev. This research was supported by a grant from the T.R. Prime Ministry State Planning Organization (Project Number 25-DPT-07 - 04-01).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Akkiliç N, Mustafaeva Z, Mustafaev M. 2007. High performance liquid chromatography study of water-soluble complexes and covalent conjugates of polyacrylic acid with bovine serum albumin. J. of Applied Polymer Science 105:3108–3120.
- Alesi S, Di Maria F, Melucci M, Macquarrie DJ, Luque R, Barbarella I. 2008. Microwave-assisted synthesis of oligothiophene semiconductors in aqueous media using silica and chitosan supported Pd catalysts. Green Chem 10:517–523.
- Budarin LV, Zhao Y, Gronnow MJ, Shuttleworth PS, Breden SW, Macquarrie DJ, Clark JH. 2011. Microwave-mediated pyrolysis of macro-algae. Green Chem 13:2330–2333.
- Cooper CL, Dubin PL, Kayitmazer AB, Turksen S. 2005. Polyelectrolyte-protein complexes. Current Opinion in Colloid & Interface Science 10:52–78.
- Dilgimen AS, Mustafaeva Z, Demchenko M, Kaneko T, Osada Y, Mustafaev MI. 2001. Water-soluble covalent conjugates of bovine serum albumin with anionic poly(N-isopropyl-acrylamide) and their immunogenicity. Biomaterials 22:2383–2392.
- Dincer B, Mustafaev MI, Bayülken S. 1997. High-performance liquid chromatography study of water-soluble ternary polyacrylamide-metal-protein complexes. J. Applied Polym. Sci 65:37–40.
- D’Souza MJA, Schowen RL, Topp EM. 2004. Polyvinylpyrrolidone-drug conjugate: Synthesis and release mechanism. J.Cont. Release 94:91–100.
- Favretto L. 2003. Milestone's microwave labstation. Molecular Diversity 7:287–291.
- Guney O, Sarac AS, Mustafaev MI. 1997. Fluorescence and turbidimetry study of complexation of human serum slbumin with polycations. Bioact. Compat. Polymers 12:231–244.
- Hermanson GT. 1996. Bioconjugate Techniques. California, CA, USA: Academic Press Inc.
- Jens W, Volodymyr B, Karl-Friedrich A. 2007. Influence of grafting on the solution properties and the dissociation behavior of ionic/nonionic grafted copolymers. Macromolecular Chemistry and Physics 208:643–650.
- Karahan M. 2009. Ph.D. thesis. Development of functional biopolymer systems containing metal. Yildiz Technical University, Esenler-Istanbul, Turkey, 1–208.
- Karahan M, Mustafaeva Z, Ozer H. 2007. Polysaccharide-protein covalent conjugates and ternary metal complexes. Asian J. Chem 19:1837–1845.
- Karahan M, Mustafaeva Z, Özeroğlu C. 2010. Investigation of ternary complex formations of polyacrylic acid with bovine serum albumin in the presence of metal ions by fluorescence and dynamic light scattering measurements. The Protein Journal 29:336–342.
- Kawaguchi S, Kitano T, Ito K, Minakata A. 1991. Sodium ion activity and electrical conductivity of poly(maleic acid) and poly(isobutylene-alt-maleic acid) in aqueous salt-free solution. Macromolecules 24:6335–6339.
- Kendrick BS, Kerwin BA, Chang BS, Philo JS. 2001. Online size- exclusion high-performance liquid chromatography light scattering and differential refractometry methods to determine degree of polymer conjugation to proteins and protein-protein or protein-ligand association states. Anal Biochem 299:136–146.
- Kitano T, Kawaguchi S, Anazawa N, Minakata A. 1987. Dissociation behavior of an alternating copolymer of isobutylene and maleic acid by potentiometric titration and intrinsic viscosity. Macromolecules 20:2498–2506.
- Ladaviere C, Delair T, Domard A, Pichot C, Mandrand B. 1999. Covalent immobilization of biological molecules to maleic anhydride and methyl vinyl ether copolymers: A physico-chemical approach. J Appl Polym Sci 71:927–936.
- Ladaviere C, Veron L, Delair T, Domard A, Pichot C, Mandrand B. 1997. Reactive polymers in diagnostics: Syntheses and characterizations of nucleic acid probes and maleic anhydride-co-methyl vinyl ether polymers. J Appl Polym Sci 65:2567–2577.
- Mansuroğlu B, Kizilbey K, Derman S, Mustafaeva Z. 2011. Investigation of protein-polyelectrolyte complex and conjugates by high performance liquid chromatography methods. Turkish Journal of Biochemistry 36:21–28.
- Minakata A, Matsumura K, Sasaki S, Ohnuma H. 1980. Potentionmetric titration of copolymers of maleic acid. Macromolecules 13: 1549–1553.
- Mustafaev M. 2004. Functionally biopolymer systems. Sigma 4:1–200.
- Mustafaev MI. 1996. Biyopolimerler (Biopolymers). Gebze/Kocaeli, Turkey: Tubitak. pp. 1–253.
- Mustafaev MI, Babakhin AA, Popov AN, Litvinov IS, Merkushov AV, Gushin IS. 1990. Influence of the structuro-chemical peculiarities of water-soluble polyelectrolyte complexes of ovalbumin on their immunological properties. Mol. Biol 24:358–369.
- Mustafaev MI, Norimov AS. 1990. Polymer-metal complexes of protein antigens: New highly effective immunogens. Biomedical Sci 1:274–278.
- Mustafaev MI, Osada Y, Matsukata M, Basalp A, Cirakoglu B, Bermek E. 1996. New amphiphilic immunogens by poly(N-isopropylacrylamide)-modified bovine serum albumin. Polym. Gels Networks 4:363–372.
- Mustafaev MI, Sarac AS. 1996. Polyelectrolyte complexes (in immunology). The Polymeric Materials Encyclopedia: Synthesis, Properties and Application. Boca Raton, FL: CRC Press Inc. pp. 5771–5777.
- Mustafaev MI, Yücel F, C rakoglu B, Bermek E. 1996. Immune response to progesterone involved in Cu2+-mediated polyanion-protein complex-antigen specificity and affinity of hybridoma clones. Immunology Letters 52:63–68.
- Neurath H, Bailey K. 1953. The Proteins. New York: Academic Press Inc. p. 630.
- Ohno N, Sugai SJ. 1990. Conformational characterization of maleic acid copolymer with an inflexible side chain. J. Macromol. Sci. Chem 27:861.
- Peters T. 1985. Serum albumin. Adv. Protein Chem 37:161–245.
- Peters T. 1996. All About albumin: Biochemistry, Genetics and Medical Applications. San Diego: Academic Press.
- Petrov RV, Mustafaev MI, Norimov AS. 1992. Physicochemical criteria for the construction of artificial immunomodulators and immunogens on the basis of polyelectrolyte complexes. Sov. Med. Rev. D. Immunol. London, UK: Harwood Academic Publishers GmbH, pp. 1–113.
- Shoukry MM, Khairy EM, El-Sherif AA. 2002. Ternary complexes involving copper(II) and amino acids, peptides and DNA constituents: The kinetics of hydrolysis of a-amino acid esters. Transition Metal Chemistry 27:656–664.
- Topuzogullar M, Cimen NS, Mustafaeva Z, Mustafaev M. 2007. Molecular-weight distribution and structural transformation in water-soluble complexes of poly(acrylic acid) and bovine serum albumin. European Polymer Journal 43:2935–2946.
- Wang N, Ye L, Zhao BQ, Yu JX. 2008. Spectroscopic studies on the interaction of efonidipine with bovine serum albumin. Braz J. Med Biol Res 41:589–595.
- Wen J, Arakawa T, Philo JS. 1996. Size-exclusion chromatography with on-line light-scattering, absorbance, and refractive index detectors for studying proteins and their interactions. Anal Biochem 240: 155–166.
- Wu XS. 2004. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers: Part III. Drug delivery application. Artificial Cells Blood Substitutes and Biotechnology 32(4):575–591.