Abstract
Prophenoloxidase (PPO) was purified from Galleria mellonella L. A 67-fold purification of the proenzyme with 352% yield was achieved by using a Sepharose 4B-L-tyrosine-p-amino benzoic acid affinity column. The purified enzyme was migrated as a single band on SDS-polyacrylamide gel electrophoresis. Km and Vmax values were 0.017 M and 1430.45 EU for catechol. Inhibition of PPO was investigated with inhibitors such as p-aminobenzoic acid, etyleneglycol, and ascorbic acid. Among them, ascorbic acid showed the strongest inhibitory activity with IC50 value of 2.94 μM. The current paper represents new strategies for the biological control of the Galleria mellonella L. insect.
Introduction
The immunity of insects consists of humoral and cellular defense actions. Humoral defense refers to antibacterial proteins and other immune-related molecules generated by the fat body and the hemocytes that are secreted into plasma to sequestrate and clear the foreign bodies from the hemocoel (Theopold et al. Citation2004). Cellular immunity includes phagocytosis, nodule formation, and encapsulation (Uçkan et Al. 2011. Several factors, including asphyxiation, the local production of cytotoxic quinones or semiquinones via the PPO activation cascade during melanization, free radicals and antibacterial peptides have been suggested to function as killing agents towards internal parasites and other foreign entities that enter the insect's haemocoel (Nappi et al. 1995, 2000, Gillespie et al. 1997, Lavine and Strand 2002, Jiravanichpaisal et al. 2006). Phenoloxidase is one of the most important enzymes that are involved in the innate immune system of insects (Cerenius and Söderhäll 2004). Phenoloxidase was also associated with other physiologically important biochemical processes such as sclerotization of the insect cuticle and wound healing (Ashida and Brey Citation1995).
Phenoloxidase (EC 1.14.18.1) is a copper-containing enzyme, widely distributed in nature, which is responsible for melanization in animals and browning in plants (Gowda and Paul Citation2002, Shelby and Popham Citation2006). PPO also catalyzes the ortho-hydroxylation of monophenols and the oxidation of o-diphenols to o-quinones (Gowda and Paul Citation2002). Phenoloxidase exists in insect hemolymph or hemocytes as a proenzyme in its inactive form called prophenoloxidase. PPO is activated by a serine proteinase (prophenoloxidase activating enzyme) cascade triggered by microbial carbohydrates such as β-1,3-glucan, peptidoglycan, and lipopolysaccharides in insects (Söderhäll and Cerenius Citation1998).
It is possible that inhibition of PPO could lead to abrogation of insect defense mechanisms and this theory is logical to regard PPO as a target of new insecticides with novel modes of action in pest control (Du et al. 2010). If the enzyme activity is inhibited or disturbed, pests may lose normal defense ability, which would be a significant method to control pests by using the PPO inhibitors (Wang et al. Citation2005, Xue et al. Citation2007, Xiao et al. Citation2008). The greater wax moth G. mellonella is an important Lepidopteran pest in beehives because it feeds on pollen and generally destroys the combs and frequently is used as a model animal for the investigation of insect immunity and host-parasitoid interactions (Uçkan et al. 2001, Er et al. 2011, Ergin et al. 2006).
To elucidate the molecular and physiological mechanisms of PPO, a detailed study of the enzyme is necessary (Beckage Citation2008). However, more attention has been paid to the investigation of PPO due to the instability and rapid loss of enzymatic activity during the purification process (Feng et al. 2008). PPO has been purified from a number of different insects, including the lepidopterans Bombyx mori (Ashida Citation1971), Manduca sexta (Aso et al. Citation1985, Hall et al. Citation1995, Jiang et al. 1997), Hyalophora cecropia (Andersson et al. Citation1989), Galleria mellonella (Kopácek et al. Citation1995), and Ostrinia furnacalis (Feng et al. 2008). The current study demonstrates the purification and characterization of PPO from G. mellonella in terms of substrate specificities, optimum pH and temperature, heat inactivation, and degrees of inhibition by general PPO inhibitors in addition to its molecular weight.
Material and Methods
Insects
Laboratory colonies of G. mellonella were established from individuals that were collected from the honeycombs maintained by beekeepers around Bal kesir, Turkey. Insects were reared at 25 ± 1°C, 60 ± 5% RH, and with a photoperiod of 12 : 12 h, L : D. The G. mellonella colony was maintained by feeding the insects with honeycomb to maintain similarity to their natural media in beehives. (Uçkan et al. 2004, 2010).
Collection of hemolymph
Last instar larvae of approximately the same sizes were used for the experiments. Larvae were precooled at 4°C, then bled by piercing the first hind leg with a sterile 19-gauge needle. Hemolymph was collected with a glass microcapillary tube (Sigma Chemical Co., St. Louis, MO) and pooled into an Eppendorf tube kept on ice.
Extraction and purification procedure
The extraction procedure was adopted from Wesche- Ebeling &Montgomery (1990). 1.7 ml hemolymph was taken in 25 ml of 0.5 M phosphate buffer (pH 7.3) containing 5% poly(ethylene glycol) and 10 mM ascorbic acid. After filtration of the homogenate through muslin, the filtrate was centrifuged at 15.000 g for 30 min, and the supernatant was collected. A crude protein precipitate was made by adding (NH4)2SO4 to 80% saturation and the precipitate was collected by centrifugation at 15.000 g for 20 min, redissolved in 5 mM phosphate buffer (pH 6.30), and dialyzed against the same buffer (Wesche-Ebeling and Montgomery Citation1990). The homogenate was purified with affinity chromotography. The affinity gel used was synthesized according to the method of Arslan and Erzengin (2004). Briefly, the enzyme solution was applied to the affinity column (1 3 10 cm), equilibrated with 0.05 M phosphate buffer (pH 5.0). The affinity gel was washed with the same buffer. PPO was eluted with the solution of 0.05 M phosphate buffer (pH 7.0) containing 1 M NaCl.
Electrophoresis
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out using the method of Laemmli (Citation1970). Samples were applied to 12% polyacrylamide gels. The slab gels of 1.5 mm thickness were run at a constant current of 180 mV. Gels were stained for protein using a standard Coomassie Blue method.
Spectrophotometric assays
Kinetic assays were carried out by measuring the increase in absorbance at 420 nm and at 25oC for catechol with a Biotek automated recording spectrophotometer. The reaction was carried out in a light path quartz cuvette. The sample cuvette contained 130 μL of substrates in various concentrations prepared in the homogenization buffer (pH 6.8) and 40 μL of the enzyme. For each measurement, the volume of solution in the quartz cuvette was kept constant at 1 mL. The reference cuvette contained all of the components except the substrate, with a final volume of 1 mL (Arslan et al. Citation1997).
Determination of protein content
The protein content was determined according to the Bradford method using bovine serum albumin as standard (Bradford Citation1976).
Enzyme kinetics and substrate specificity
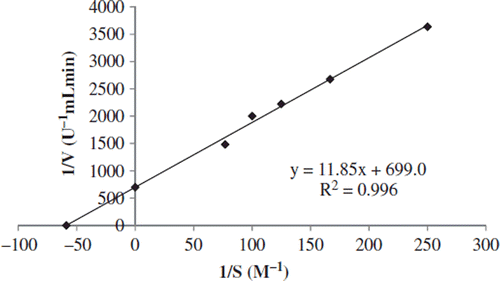
PPO activity was assayed using catechol as substrate. The rate of the reaction was measured in terms of the increase in absorbance at the absorption maxima of the corresponding quinone products for catechol substrate. Enzyme activity was calculated from the linear portion of the curve. One unit of PPO activity was defined as the amount of enzyme that causes an increase in absorbance of 0.001 unit/min for 1 ml of enzyme at 25°C (Arslan et Al. 2004). Catechol substrate, Michaelis-Menten constant (Km), and maximum velocity (Vmax) were determined according to the method of Lineweaver-Burk.
Inhibition of PPO activity
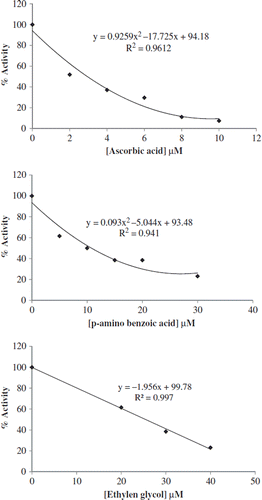
IC50 values of different inhibitors p-aminobenzoicacid, etyleneglycol, and ascorbic acid were determined on PPO. In order to determine IC50 values, 0.1 M catechol was used as substrate. At first the activity of enzyme was assay without inhibitor. This measure was accepting 100% activity for graph and than enzyme activity assay with different inhibitor concentration. In order to determine IC50 values were drawn by using a % activity-inhibitor concentration graph. IC50 values were determined from these graphs.
Results and Discussion
Extraction and purification of PPO
In this study, Galleria mellonella L. PPO was purified with affinity chromatography. The purification procedures are summarized in . As seen in , PPO was purified up to 67.2-fold. Different purification protocols have been used for PPO enzymes from different sources (Jiang et al. 1998). In order to purify PPO from different sources, we used Triton X-100, ammonium sulfate precipitation, dialysis, affinity chromatography, sephadex G-200, and Phenyl sepharose hydrophobic chromatography (Arslan et al. Citation2004, Jiang Citation1999, Weemes et al. 1998). However, pear PPO was purified generally in two or more steps asTriton X-100, ammonium sulfate precipitation, dialysis and acetone precipitation (Siddiq and Cash Citation2000, Weemes et al. 1998, Carbonaro and Mattera Citation2001).
Table I. Purification of polyphenol oxidase from Galleria Mellonella L.
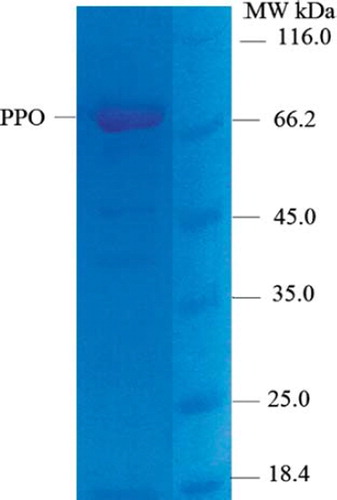
The molecular weight of PPO was estimated on SDS-PAGE with a single band of approximately 66.2 kDa (). The molecular mass of PPO from other species has been reported as follows: Sunn pest Eurygaster integriceps Puton (Hemiptera: Scutelleridae), 22 kDA (Aras et al. 2011), Hyalophora cecropia, 76 kDA (Andersson et al. Citation1989).
Substrate specificity and enzyme kinetics
PPO activity in purified enzyme was examined with regard to its diphenolase activities. The substrate specificity of the enzyme was investigated using one chemical name of catechol as substrate. The Lineweaver-Burk plot analysis of this enzyme preparation showed Km value of 0.017 M for catechol and Vmax value was 1430.45 (U/mL.min.) for catechol (, ). Km of 2.25, 1.35, 1.30, and 0.17 mM were reported for Heliothis virescens Fabricius (Lepidoptera: Noctuidae) (Lockey and Ourth Citation1992), Spodoptera littoralis Fabricius (Lepidoptera: Noctuidae) (Lee and Anstee Citation1995), Drosophila melanogaster L. (Diptera: Drosophilidae) (Asada and Sezaki Citation1999), and Apis mellifera L. (Hymenoptera: Apididae) (Zufelato et al. Citation2004), respectively. The Km of Galleria mellonella L. PO is higher than that of other insects, indicating the lower affinity of the enzyme to catechol.
Table II. Kinetic values of Galleria Mellonella L.
The Vmax/Km ratio is called the “catalytic power,” and it is a better parameter to find the most effective substrate (Dogan et al. 2000). The ratio Vmax/Km is 84144. The large ranges in the apparent Km values of PPO reported may be due to different reasons: different assay methods used for different varieties, different origins of the same variety, and different values of pH of extraction (Rocha et al. Citation1998).
Inhibition of PPO
Inhibition of PPO by ascorbic acid, p-aminobenzoic acid, and ethyleneglycol has been investigated. It was found that the presence of all chemicals causes the inhibition of PPO. shows the effect of ascorbic acid, p-aminobenzoic acid, and ethyleneglycol inhibitor on PPO using catechol as substrate. The enzymatic browning by a specific inhibitor may involve a single mechanism or may be the result of the interplay of two or more mechanisms of inhibitor action. Inhibition of PPO was investigated with inhibitors such as ascorbic acid, p-aminobenzoicacid, and etyleneglycol using catechol as substrate. Among them, ascorbic acid showed the strongest inhibitory activity with IC50 value of 2.94 μM. Ethylen glycol was a poor effective inhibitor for PPO (IC50 25.44 μM) ().
Table III. The values IC50 of some compounds on Galleria Mellonella L. PPO.
All in all, the present article reports for the first time the isolation and qualitative characterization of the PO enzyme in the Galleria mellonella L. pest. In consideration of the role played by PO in the insect immunity, this information could be profitable not only to comparative biochemists but also to researchers that see in the immune system a possible target for biological control of insect pests.
Declaration of interest
The authors report no conlicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Amiot MJ, Tacchini M, Aubert SY, Oleszek W. 1995. Influence of cultivar, maturity stage, and storage-conditions on phenolic composition and enzymatic browning of pear fruits. Journal of Agricultural and Food Chemistry 43(5):1132–1137.
- Andersson K, Sun S-C, Boman HG and Steiner H. 1989.Purification of the propbenoloxidase from Hyalophora cecropia and four proteins involved in its activation. Insect Biochem. 19:629–637.
- Arslan O, Erzengin M, Sinan S, Ozensoy O. 2004. Purification of mulberry (Morus alba L.) polyphenol oxidase by affinity chromatography and investigation of its kinetic and electrophoretic properties. Food Chemistry, 88(3):479–484.
- Arslan O, Temur A, Tozlu I. 1997. Polyphenol oxidase from Allium sp. J. Agric. Food Chem. 45:2861–2863.
- Arslan O, Tozlu I. 1997. Substrate specificity, heat inactivation and inhibition of polyphenol oxidase from Anethum graveolens L. Ital. J. Food Sci. 9(3):249–253.
- Asada N, Sezaki H. 1999. Properties of phenoloxidases generated from prophenoloxidase with 2-propanol and the natural activator in Drosophila melanogaster. Biochem. Genet. 37:149–158.
- Ashida M. 1971. Purification and characterization of pre- phenoloxidase from the hemolymph of the silkworm, Bombyx mori. Arch. Biochem. Biophys. 144:749–762.
- Ashida M, Brey PT. 1995. Role of the integument in insect defense: Prophenoloxidase cascade in the cuticular matrix. Proceed Nat Acad Sci USA Immunol, 92:10698–10702.
- Aso Y, Kramer KJ, Hopkins TL, Lookhart GL. 1985. Characterization of hemolymph protyrosinase and a cuticular activator from Manduca sexta (L). Insect Biochem 15:9–17.
- Augustin MA, Ghazali HM, Hashim H. 1985. Polyphenol oxidase from guava (Psidium guajaVa L.). J. Agric. Food Chem. 36:1259–1265.
- Beckage NE. 2008. Insect immunology: Purification and characterization of hemolymph prophenoloxidase from Ostrinia furnacalis (Lepidoptera: Pyralidae) larvae. Congjing Feng, Qisheng Song b, Wenjing Lü, Jianfeng Lu, eds. Comparative Biochemistry and Physiology, Part B 151:139–146.
- Benjar C, Athapol N. 2006. Thermal inactivation of polyphenoloxidase in pineapple puree. LWT 39:492–495.
- Blumenthal M, Goldberg A, Brinckman J. 2000. Herbal Medicine: Expanded Commission E Monographs. Austin: American Botanical Council.
- Bradford M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Athens, GA: Reproduction Research Laboratories, Dept. of Biochemistry, University of Georgia.
- Carbonaro M, Mattera M. 2001. Polyphenoloxidase activity and polyphenol levels in organically and conventionally grown peach (Prunus persica L., cv. Regina bianca) and pear (Pyrus communis L., cv. Williams). Food Chemistry, 72:419–424.
- Cerenius L, Söderhäll K. 2004. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 198:116–126.
- Davis R, Pierpoint WS. 1975. Problem of the reactive species from enzymic and chemical oxidation of o-diphenols: Anomalies in the trapping of o-quinonoids with benzenesulfinic acid. Biochem. Soc. Trans. 3:671.
- Dogan M, Arslan O, Dogan S. 2002. Substrate specificity, heat inactivation and inhibition of polyphenol oxidase from differentaubergine cultivars. Int. J. Food Sci. Technol. 37:415–423.
- Dogan S, Arslan O, Ozen F. 2005. Polyphenol oxidase activity of oregano in different stages. Food Chem. 91:341–345.
- Dogan S, Dogan M, Arslan O. 2003. Characterization of polyphenol oxidase from Thymus (Thymus longicaulis var. subisophyllus). Adv. Food Sci. 25(2):56–64, 3509–3517.
- Er A, U kan F, Rivers DB, Sak O. 2011. Cytotoxic effects of parasitism and application of venom from the endoparasitoid Pimpla turionellae on hemocytes of the host Galleria mellonella. J. Appl. Entomol. 135:225–236.
- Ergin E, U kan F, Rivers DB, Sak O. 2006. In vivo and in vitro activity of venom from the endoparasitic wasp Pimpla turionellae (L.) (Hymenoptera: Ichneumonidae). Arch. Insect Biochem. 61:87–97.
- Erat M, Şakiroglu H, Kuvrevioglu OI. 2006. Purification and characterization of polyphenol oxidase from Ferula sp. Food Chemistry 95:503–508.
- Flurkey WH, Jen JJ. 1980. Purification of peach polyphenoloxidase in the presence of added protease inhibitors. J. Food Biochem. 4:829.
- Flurkey WH. 1989. Polypeptide composition and aminoterminal sequence of broad bean polyphenoloxidase. Plant Physiology, 91:481–483.
- Galeazzi MAM, Sgarbieri VC, Constantinides SM. 1981. Isolation, purification and physicochemical characterization of polyphenoloxidase (PPO) from a dwarf variety of banana (Musa caVendishii, L). J. Food Sci. 46:150–155.
- Golbeck JH, Cammarata KV. 1981. Spinach thylakoid polyphenol oxidase: Isolation, activation and properties of the native chloroplast enzyme. Plant Physiology, 67:877–884.
- Gowda LR, Paul B. 2002. Diphenol activation of the monophenolase and diphenolase activities of field bean (Dolichos lablab) polyphenol oxidase. J. Agric. Food Chem. 50:1608–1616.
- Gunata YZ, Sapis JC, Moutonet M. 1987. Substrates and aromatic carboxylic and inhibitors of grape polyphenoloxidases. Phytochemistry 26:1573–1575.
- Hall M, Scott M, Sugumaran M, Söderhäll K, Law JH. 1995. Proenzyme of Manduca sexta phenoloxidase: Purification, activation, substrate specificity of the active enzyme and molecular cloning. Proc Natl Acad Sci USA 92:7764–7768.
- Huntcheson SW, Buchanan BB. 1980. Polyphenol oxidation by Vicia faba chloroplast membranes. Plant Physiology, 66:1150–1154.
- Janovitz-Klapp AH, Richard FC, Goupy PM, Nicolas JJ. 1990. Kinetic studies on apple polyphenol oxidase. J. Agric. Food Chem. 38:1437–1441.
- Jiang YM. 1999. Purification and some properties of polyphenol oxidase of longan fruit. Food Chemistry, 66:75–79.
- Jiang H, Wang Y, Ma C, Kanost MR. 1997a. Subunit composition of prophenoloxidase from Manduca sexta: Molecular cloning of subunit ProPo-P1. Insect Biochem Mol Biol 27:835–850.
- Kahn V, Andrawis A. 1985. Inhibition of mushroom tyrosinase by tropolone. Phytochemistry 24:905–908.
- Kermasha S, Goetghebeur M, Monfette A. 1993. Studies on inhibition of mushroom polyphenol oxidase using chlorogenic acid as substrate. J. Agric. Food Chem. 41:526–531.
- Kim MJ, Kim CY, Park I. 2005. Prevention of enzymatic browning of pear by onion extract. Food Chemistry, 89(2):181–184.
- Kopácek P, Weise C, Gotz P. 1995. The prophenoloxidase from the wax moth Galleria mellonella: Purification and characterization of proenzyme. Insect Biochem Mol Biol 25:1081–1091.
- Kowalski SP, Eannetta NT, Hirzei AT, Steens JC. 1992. Purification and characterization of polyphenol oxidase from glandular trichomes of Solanum berthaultii. Plant Physiology, 100:677–684.
- Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685.
- Lamikandra O, Sharon DK, Mitwe NM. 1992. Muscadine grape polyphenol oxidase: Partial purification by high-pressure liqid chromatography and some properties. Journal of Food Science, 57:686–689, 695.
- Lattanzio V, Cardinall A, Di Venere D, Linsalata V, Palmieri S. 1994. Browning phenomena in stored artichoke (Cynara scolymus L.) heads: Enzymic or chemical reactions?. Food Chem. 50:1–7
- Lee CY, Smith NL, Pennesi AP. 1983. Polyphenol oxidase from DeChaunac grapes. J. Sci. Food Agric. 34:987–991.
- Lee CY, Whitaker JR. 1995. Enzymatic Browning and its Prevention. Washington, DC: American Chemical Society.
- Lee MJ, Anstee JH. 1995. Phenoloxidase and its zymogen from haemolymph of larvae of lepidopteran Spodoptera littoralis (Lepidoptera: Noctuidae). Comp. Biochem. Physiol. B 110, 379–384.
- Lockey TD, Ourth DD. 1992. Isolation and characterization of hemolymph phenoloxidase from Heliothis virescens larvae. Comp. Biochem. Physiol. B. 102, 891–896
- Marshall MR, Kim J, Wei C. 2000. Enzymatic browning in fruits, vegetables and seafoods. Available at http://www.fao.org/ag/Ags/agsi/ENZYMEFINAL/Enzymatic%20Browning.html.
- Murata M, Tsurutani M, Tomita M, Homma S, Kaneko K. 1995. Relationship between apple ripening and browning: Changes in polyphenol content and polyphenol oxidase. J. Agric. Food Chem. 43:1115.
- Nilo D, Rivas J, Whitaker JR. 1973. Purification and some properties of two polyphenol oxidases from bartlett pear. Plant Physiol. 52: 501–507.
- Nishimura M, Fukuda C, Murata M, Homma S. 2003. Cloning and some properties of Japanese pear (Pyrus pyrifolia) polyphenol oxidase, and changes in browning potential during fruit maturation. Journal of the Science of Food and Agriculture 83(11):1156–1162.
- Nünez-Delicado E, Sanchez-Ferrer A, Garcia-Carmona FF, Lopez-Nicolas JM. 2005. Effect of organic farming practices on the level of latent polyphenol oxidase in grapes. J. Food Sci. 70(1):74–85.
- Pifferi PG, Baldassari L, Cultrera R. 1974. Inhibition by carboxylic acids of an o-diphenol oxidase from Prunus avium fruits. J. Sci. Food Agric. 25:263–270.
- Pizzocaro F, Torreggiani D, Gilardi G. 1993. Inhibition of apple polyphenol oxidase by ascorbic acid, citric acid and sodium chloride. Journal of Food Processing and Preservation 17:21–30.
- Robert C, Rouch C, Cadet F. 1997. Inhibition of palmito (Acanthophoenix rubra) polyphenol oxidase by carboxylic acids. Food Chem. 59(3):355–360.
- Rocha AMCN, Pilar-Cano M, Galeazzi MAM, Morais AMMB. 1998. Characterization of ‘‘Starking” apple polyphenoloxidase. J. Sci. Food Agric. 77:527–534.
- Roudsari MH, Signoset A, Crovzet J. 1981. Eggplant polyphenol oxidase: Purification, characterization and properties. Food Chem. 7:227–235.
- Sakiroglu H, Küfrevioglu IO, Kocacaliskan I, Oktay M, Onganer Y. 1996. Purification and characterization of Dog-rose (Rosa dumalis Rechst.) polyphenol oxidase. J. Agric. Food Chem. 44:2982–2986.
- Schwartz B, Olgin AK, Klinman JP. 2001. The role of cooper in topa quinone biogenesis and catalysis, as probed by azide inhibiton of a copper amine oxidase from yeast. Biochemistry 40:2954–2963.
- Shelby KS, Popham HJR. 2006. Plasma phenoloxidase of the larval tobacco budworm, Heliothis virescens, is virucidal. J. Insect Sci. 13:2442–2448.
- Siddiq M, Cash JN. 2000. Physico-chemical properties of polyphenol oxidase from d’Anjou and Bartlett pears (Pyrus communis L.). Journal of Food Processing and Preservation 24 (5):353–364.
- Söderhäll K, Cerenius L. 1998. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr. Opin. Immunol. 10:23–28.
- Takeo T, Baker JE. 1972. Changes in multiple forms of polyphenol oxidase during maturation of tea leaves. Phytochemistry, 12: 21–24.
- Theopold U, Schmidt O, Soderhall K, Dushay MS. 2004. Coagulation in arthropods, defence, wound closure and healing. Trends Immunol. 25:289–294.
- Tono T. 1995. Purification and properties of polyphenol oxidase from cabbage (Brassica oleracea L.). Journal of Agricultural Food Chemistry, 43:1138–1142.
- U kan F, Sinan S, Savas S, Ergin E. 2004. Determination of venom components from the endoparasitoid wasp Pimpla turionellae L. (Hymenoptera; Ichneumonidae). Ann. Entomol. Soc. Am. 97: 775–780.
- U kan F, Er A, Ergin E. 2010. Levels of encapsulation and melanization in Galleria mellonella (Lepidoptera: Pyralidae) parasitized and envenomated by Pimpla turionellae (Hymenoptera: Ichneumonidae). J. Appl. Entomol. 134:718–726.
- Walker JRL, Wilson EL. 1975. Studies on the enzymatic browning of apples: Inhibition of apple o-diphenol oxidase by phenolics acids. J. Sci. Food Agric. 26:1825–1831.
- Wang SD, Luo WC, Xu SJ, Ding Q. 2005. Inhibitor effects of 4-dodecylresrcinol on the phenoloxidase of the diamondback moth Plutella xylostella (L.) (Lepidoptera: Plutellidae). Pest. Biochem. Physiol. 82:52–58.
- Wesche-Ebeling P, Montgomery MW. 1990. Strawberry polyphenol oxidase: Extraction and partial characterization. J. Food Sci. 55:1320–1325.
- Wissemann KW, Lee CY. 1981. Characterization of polyphenoloxidase from Ravat 51 and Nigara grapes. J. Food Biochem., 46:506.
- Wissemann KW, Lee CY. 1985. Characterization of apolyphenol oxidase from Ravat 51 and Niagara grapes. Journal of Food Science, 46:506–508, 514.
- Xiao T, Xie XY, Xue CB, Luo WC, Jiang L, Cao S. 2008. Inhibitory effects of Schiff analogs of salicylidene aniline on phenoloxidase from Pieris rapae L.(Lepidoptera: Pieridae). Pest. Biochem. Physiol. 91:39–44.
- Xue CB, Luo WC, Jiang L, Xie XY, Xiao T, Yan L. 2007. Inhibition kinetics of cabbage butterfly (Pieris rapae L.) larvae phenoloxidase activity by 3-hydroxy-4-methoxybenzaldehyde thiosemicarbazone. Appl. Biochem. Biotechnol. 143:101–114.
- Yang CP, Fujita S, Ashrafuzzaman MA, Nakamura N, Hayashi N. 2000. Purification and chracterization of polyphenol oxidase from banana (Musa sapientum L.) pulp. Journal of Agricultural Food Chemistry, 48:2732–2735.
- Zawistowski J, Biliaderis CG, Murray ED. 1988a. Purification and characterization of Jerusalem artichoke (Helianthus tuberosus L.) polyphenol oxidase. Journal of Food Biochemistry, 12:1–22.
- Zawistowski J, Biliaderis CG, Murray ED. 1988b. Isolation and some properties of an acidic fraction of polyphenol oxidase from Jerusalem artichoke (Helianthus tuberosus L.). Journal of Food Biochemistry, 12:23–35.
- Zibaee A, Bandani AR, Malagoli D. 2011. PuriFIcation and characterization of phenoloxidase from the hemocytes of Eurygaster integriceps (Hemiptera: Scutelleridae). Comparative Biochemistry and Physiology, Part B. 158:117–123
- Zufelato MS, Lourenco AP, Simões ZL, Jorge JA, Bitondi MM. 2004. Phenoloxidase activity in Apis mellifera honey bee pupae, and ecdysteroid-dependent expression of the prophenoloxidase mRNA. Insect Biochem. Mol. Biol. 34:1257–1268.