Abstract
Paraoxonase (PON) was purified and characterized from the Merino and Kivircik sheep's blood serums by a two-step procedure using ammonium sulphate precipitation and Sepharose-4B-L-tyrosine-1-napthylamine hydrophobic interaction chromatography for the first time. On SDS-polyacyrilamide gel electrophoresis, purified human serum paraoxonase yielded a single band of 66 kDa on SDS-PAGE. The KM and Vmax were 0.482 mM and 41.348 U/mL.dak for Merino PON enzyme, 0.153 mM and 70.289 U/mL.dak for Kivircik PON, respectively. The effect of Mn2+ , Hg2+ , Co2+ , Cd2+ , Ni2+ and Cu2+ heavy metals on purified Merino and Kivircik serum PON in vitro was determined.
Keywords::
Introduction
Paraoxonase (EC 3.1.8.1, PON1) is a calcium-dependent serum esterase that is synthesized by the liver. In serum, it is closely associated with high-density lipoproteins (Aldridge Citation1953). Paraoxonase hydrolyze organophosphate compounds are widely used as insecticides and nerve gases. Therefore, PON1 plays a major role in the detoxification of these compounds and other artificial substrates, so that it may alter significantly an individual's susceptibility to the toxicity of these chemicals. In addition, paraoxonase is involved in lipid metabolism, since this enzyme probably hydrolyzes multiple oxygenated forms of polyunsaturated fatty acids of low-density lipoproteins associated with phospholipids. For this reason, paraoxonase can be defined as an antioxidant enzyme (Watson et al. Citation1995a, Citation1995b). The physiological substrates of PON1 are still unknown, but structure-reactivity studies (Aviram et al. Citation1998) and laboratory evolution experiments (Getz and Reardon Citation2004) indicate that the native activity of PON1 is lactonase. PON1 hydrolyzes a wide range of substrates, such as esters, thioesters, phosphotriesters, carbonates, lactones, and thiolactones. The highest activities observed thus far are with synthetic substrates such as phenyl acetate and dihydrocoumarin (Draganov and La Du Citation2004, Aharoni et al. Citation2004) that have no physiological relevance. It is therefore unlikely that these are PON1's native substrates. Recently, lactonase (lactone hydrolysis) as well as lactonizing (lactone formation) activities of PON1 were described, including those with lactones of potential physiological relevance such as products of fatty acid oxidation (Billecke et al. Citation2000, Teiber et al. Citation2003).
These results imply that PON1 might, in fact, be a lactonase rather than an aryl-esterase or paraoxonase, as traditionally described.
Different sheep breeds used in this were Karacabey Merino and Kivircik. The main native sheep breed of the Thrace and Marmara regions of Turkey is Kivircik (Kaymakci Citation2006). The German Mutton Merino was brought into Turkey in the 1930s to increase live weight and fleece quality of indigenous sheep breeds (Koyuncu and Uzun Citation2009). The Karacabey Merino was obtained by crossbreeding the German Mutton Merino with indigenous Kivircik sheep at the Karacabey State Farm (Yalcin Citation1986). It has been demonstrated that serum PON1 activity was reduced in early postpartum dairy cows (Turk et al. Citation2004).
Metals are notable for their wide environmental dispersion from such activity, their tendency to accumulate in tissues of the human body, and their overall potential to be toxic even at relatively minor levels of exposure. Some metals, such as copper and iron, are essential to life and play irreplaceable roles in, for example, the functioning of critical enzyme systems. Other metals are xenobiotics, i.e., they have no useful role in human physiology (and most other living organisms) and, even worse, as in the case of lead and mercury, may be toxic even at trace levels of exposure (Hu and McCally 2002).
The main goal of this study is to purify the PON enzyme from Merino and Kivircik sheep's blood serums using simple and cheap methods and observe kinetic alterations in the enzyme activity in the presence of metal ions, including Mn2+ , Hg2+ , Co2+ , Cd2+ , Ni2+ and Cu2+ . The rationale to perform this study is that exposure to heavy metals is an important problem of environmental toxicology. Most heavy metals are toxic to humans, animals, and plants, and man is at great risk of suffering from health hazards associated with toxic metals because of bioaccumulation (Hura and Hura Citation2006).
Materials and Methods
Materials
The materials used include paraoxon and protein assay reagents obtained from Sigma Chem. Co and Merck. All other chemicals used were of reagent grade. The blood samples were collected from two different sheep breeds in Balikesir, Turkey.
Collection of blood samples
The blood samples were collected from each sheep breed within dry tubes. For preparation of serum, the tubes were centrifuged at 5.000 rpm for 10 min and the serum was removed. Serum was used for all enzyme assays.
Paraoxonase enzyme assay
Paraoxonase enzyme activity towards paraoxon was quantified spectrophotometrically by the method described Gan et al. (Citation1991). The reaction was followed for 2 min at 37°C by monitoring the appearance of p-nitrophenol at 412 nm in Biotek automated recording spectrophometer. About 2 mM final substrate concentration was used during enzyme assay and all measurements were taken in duplicate and corrected for the non-enzymatic hydrolysis. One enzyme unit was defined as the amount of enzyme that catalyzes the hydrolysis of 1µmol of substrate at 37°C.
Ammonium sulphate precipitation
Triton X-100-treated serum was precipitated with ammonium sulphate. The precipitation intervals for PON were 60–80% (Sinan et al. Citation2006). The precipitate was collected by centrifugation at 15,000 rpm for 20 min and redissolved in 100 mM Na-phosphate buffer (pH7.0).
Synthesis of sepharose 4B-L-tyrosine-1-naphtylamine hydrophobic interaction gel
Sepharose-4B was activated by CNBr. After that, L-tyrosine was attached to the activated gel as a spacer arm, and finally, diazotized naphthylamine was clamped to the para position of aniline molecule as ligand. Thus, Sepharose 4B-aniline-1-naphtylamine hydrophobic interaction gel was obtained. The chromatography column was equilibrated with 100 mM phosphate buffer pH 8, including NaCl (Sinan et al. Citation2006).
Preparation of hydrophobic interaction chromatography column
Precipitate from the ammonium sulphate was loaded onto the Sepharose 4B-aniline-1-naphtylamine hydrophobic interaction column, which had been equilibrated with 100 mM Na-phosphate buffer (pH 8.0). Elution was performed using NaCl gradient. Fractions were analyzed for both protein amount (280 nm) and enzyme activity (412 nm). Tubes with enzyme activity were combined for other kinetic studies.
Protein determination
During the purification steps, protein quantity was determined spectrophotometrically at 595 nm according to the Bradford method using bovine serum albumin as the standard (Bradford Citation1976).
SDS-polyacrylamide gel electrophoresis
Sodium dodecyl sulphate (SDS)-polyacrylamide gel electrophoresis (PAGE) was performed after purification of the enzyme. It was performed with 10% and 3% acrylamide concentrations for the separating and stacking gels, respectively, and 0.1% SDS (Laemmli Citation1970). The sample (20 µg) was applied to the electrophoresis medium. Gels were stained for 1.5 h in 0.1% Coomassie Brilliant Blue R-250, 50% methanol, and 10% acetic acid, then destained with several changes of the same solvent without dye. The electrophoretic pattern was photographed.
Kinetic studies and determined of KM and Vmax values
For the kinetic studies of PON enzyme activity, different concentrations of substrate were added to the reaction medium. PON activity was assayed by following the hydration of paraoxon. In order to obtain Km and Vmax values, the enzyme using paraoxon as a substrate was measured at seven different substrate concentrations at pH 8.0 and 37°C. Km and Vmax values were determined by means of Lineweaver-Burk graphs (Lineweaver and Burk Citation1934).
In vitro metal studies
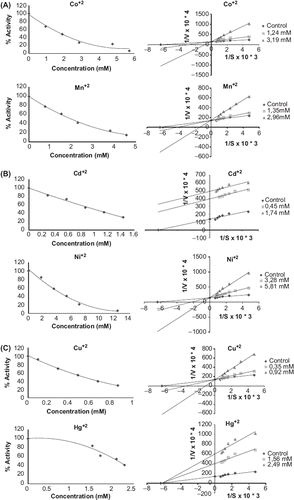
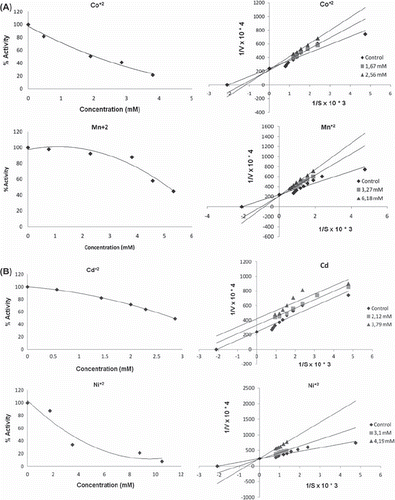
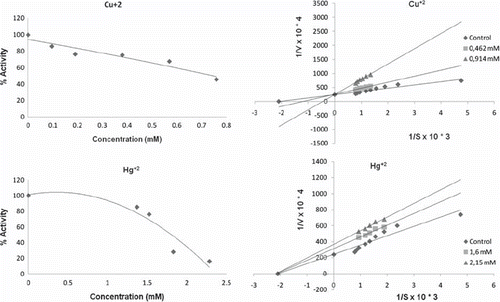
PON enzyme activities were measured in the presence of Mn2+ , Hg2+ , Co2+ , Cd2+ , Ni2+ and Cu2+ at different cuvette concentrations. Control activity was assumed to be 100% in the absence of inhibitor. For each metal ion, a percent activity versus metal ion concentration graph was drawn. For the metals having an inhibition effect, the inhibitor concentration causing up to 50% inhibition (IC50 values) was determined from the graphs. For determination of Ki values, three different inhibitor concentrations were tested for each metal ion. In these experiments, paraoxon was used as substrate at five different concentrations (0.15, 0.3, 0.45, 0.6, and 0.75 mM). Lineweaver–Burk curves (1934) were used for determination of Ki and inhibitor type.
Results
Purification of human paraoxonase from Merino and Kivircik sheep's blood serums
Merino and Kivircik sheep's blood serums paraoxonase was purified by two sequential procedures, ammonium sulphate precipitation followed by hydrophobic interaction chromatography, specifically designed for a PON enzyme. This method is quite cheap and easy to design, as well as taking a very short time.
The enzyme activity and total protein concentration were determined from all fractions collected from each purification step. Merino PON 462.7-fold and Kivircik PON 461.7-fold were purified (). The final purified PONs had only one protein band on SDS-PAGE, with a molecular weight of 66 kDa ().
Figure 1. SDS-PAGE of merino and kivircik serum paraoxonase. The pooled fractions from ammonium sulfate precipitation and hydrophobic interaction chromatography (sepharose-4B, L-tyrosine, 1-napthylamine) were analyzed by SDS-PAGE (12% and 3%) and revealed by Coomassie Blue staining. Experimental conditions were as described in the method. Lane 3 contained 3 μg of various molecularmass standards: ß-galactosidase (116.0), bovine serum albumin (66.0), ovalbumin (45.0), lactate dehydrogenase (35.0), ∞-lactoglobulin (25.0), lysozyme (19.5).
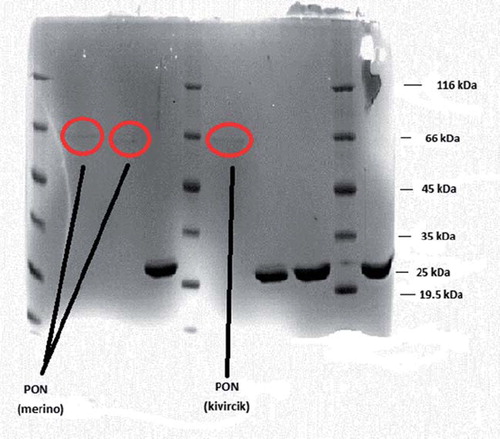
Table I. Summary of the purification of different sheep serum paraoxonase.
Inhibitory effects of metal ions on Merino and Kivircik sheep's PON activity
The kinetic parameters for the various metal chlorides are presented in . The IC50 values obtained with purified Merino and Kivircik PON enzyme are in different value ( and ). The metals in both Merino and Kivircik PON enzyme were found to be the most powerful inhibitory. The next step was to study the kinetics of interaction of heavy metals with the purified Merino and Kivircik PON enzyme. Two different concentrations of heavy metals were used for the determination of inhibition types (, ). Inhibition properties of purified Merino and Kivircik PON enzyme solution by Mn2+ , Hg2+ , Co2+ , Cd2+ , Ni2+ and Cu2+ were investigated with paraoxon as substrate at pH 8.0 (). Metal ions exhibit inhibitory effects on Merino PON1 and Kivircik at low concentrations with IC50 values ranging from 0.747to 5.140 mM and 0.530 to 4.220, respectively. The inhibitory constants (Ki) of the metal ions are presented in . Cu+ 2 was the most powerful inhibitor among others ( and ).
Table II. Type of inhibition and IC50 values (mM) of metals on merino and kivircik paraoxonase enzyme.
Discussion
The main goal of this study is to purify PON enzyme from Merino and Kivircik sheep's blood serums using simple and cheap methods and observe kinetic alterations in the enzyme activity in the presence of metal ions, including Mn2+ , Hg2+ , Co2+ , Cd2+ , Ni2+ and Cu2+ . We purified the enzyme with high specific activity and observed that metals inhibit the pure enzyme at low concentrations.
Many chemicals influence metabolism at low concentrations by decreasing or increasing normal enzyme activity, especially by inhibiting specific enzymes with critical function, and they are important drug targets (Ekinci and Beydemir Citation2009).
Over the past years, undesired impacts of metal ions on various enzymes have been increasingly reported. For instance, Ekinci et al. (Citation2007) reported the inhibitory effects of heavy metal ions incorporating lead, cobalt, and mercury on the activity of cytosolic human carbonic anhydrase isoenzymes I and II. It was determined that those heavy metals exhibited different inhibition mechanisms at different concentrations. Another investigation then came regarding the interactions between rainbow trout carbonic anhydrase and metal ions, including cobalt, copper, zinc, silver, and cadmium. The metals decreased the activity at low concentrations (Soyut et al. Citation2008). In addition to the studies above, there are investigations regarding the inhibitory effects Please close up space hereof metals on PON activity as well. We investigated the in vitro effects of some heavy metals (Hg, Cd, Cu, Mn, and Ni) on the purified human serum PON1Q and R isoenzyme. In that study, metals were found to be more effective inhibitors on purified human serum PON1(R192) activity than PON1(QI92) activity (Gencer and Arslan Citation2009).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
Notice of Correction
The version of this article published online ahead of print on 04 Sept 2012 contained an error on page 6, The sentence “In addition to the studies above, there are investigations regarding the inhibitory effects Please close up space hereof metals on PON activity as well.” should have read “In addition to the studies above, there are investigations regarding the inhibitory effects of metals on PON activity as well.”. The error has been corrected for this version.
References
- Aharoni L, Gaidukov S, Yagur L . 2004. Directed evolution of mammalian paraoxonases PON1 and PON3 for bacterial expression and catalytic specialization. Proc. Natl. Acad. Sci. U. S. A. 101:482–487.
- Aldridge WN. 1953. Serum esterases. 1. Two types of esterase (A and B) hydrolysing p-nitrophenyl acetate, propionate and butyrate, and a method for their determination. Biochem. J. 53:110–117.
- Aviram M, Rosenblat M, Bisgaier CL . 1998. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions: A possible peroxidative role for paraoxonase. J. Clin. Invest. 101:1581–1590.
- Billecke S, Draganov D, Counsell R, Stetson P, Watson C, Hsu C, La Du BN. 2000. Drug Metab. Dispos. 28:1335.
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–251.
- Draganov DI, La Du BN. 2004. Pharmacogenetics of paraoxonases: A brief review. Naunyn Schmiedebergs Arch Pharmacol. 369:78–88.
- Ekinci D, and Bedemir S. 2009. Evaluation f the impact of antibiotic drugs on PON1: A major bioscavenger against cardiovascular disease. Eur. J. Pharmacol., 617:84–89.
- Ekinci D, Beydemir S, Kufrevioglu OI. 2007. In vitro inhibitory effects of some heavy metals on human erythrocyte carbonic anhydrases. J Enzyme Inhib Med Chem 22:745–750.
- Gan KN, Smolen A, Eckerson HW, La Du BN. 1991. Purification of human serum paraoxonase/arylesterase, evidence for one esterase catalyzing both activities. Drug Metab. Dispos., 19:100–106.
- Gencer N, Arslan O. 2009. Purification human PON1(Q192) and PON1(R192) isoenzymes by hydrophobic interaction chromatography and investigation of the inhibition by metals. J Chromatogr B 877:134–140.
- Getz GS, and Reardon CA. 2004. Paraoxonase, a cardioprotective enzyme: Continuing issues. Curr Opin Lipidol. 15:261–267.
- Hu H, McCally M (eds.) 2002. Life support: The environment and human health (book review). J Sociol Soc Welf 4:1–9.
- Hura C, Hura BA. 2006. Assessment of the heavy metals in the food from Romania, 2005. Misc Toxicol Lett 164:270.
- Kaymakci M. 2006. Advanced Sheep Breeding, Sheep and Goat Breeds Assoociation, İzmir, Turkey, 49–77.
- Koyuncu M, Uzun SK. 2009. Growth performance of Karacabey Merino and Kivircik lambs under semi-intensive management in Turkey. Small Ruminant Res., 83:64–66.
- Laemmli DK. 1970. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680–683.
- Lineweaver H, Burk D. 1934. The determination of enzyme dissociation constants. J Am Chem Soc 57:685.
- Sinan S, Kockar F, Arslan O. 2006. Novel purification strategy for human PON1 and inhibition of the activity by cephalosporin and aminoglycoside derived antibiotics. Biochimie 88:565–574.
- Soyut H, Beydemir S, Hisar O. 2008. Effects of some metals on carbonic anhydrase from brains of rainbow trout. Biol Trace Elem Res 123:179–190.
- Teiber JF, Draganov DI, La Du BN. 2003. Biochem. Pharmacol. 66:887.
- Turk R, Juretic D, Geres D, Turk N, Rekic B, Simeon-Rudolf V, and Svetina A. 2004. Serum paraoxonase activity and lipid parameters in the early postpartum period of dairy cows. Res. Vet. Sci., 76: 57–61.
- Watson AD, Berliner JA, Hama SY . 1995a. Protective effect of high density lipoprotein associated paraoxonase: inhibition of the biological activity of minimally oxidized low-density lipoprotein. J Clin Invest. 96:2882–2891.
- Watson AD, Navab M, Hama SY . 1995b. Effect of platelet activating factoracetylhydrolase on the formation and action of minimally oxidized low density lipoprotein. J Clin. Invest. 95b:774–782.
- Yalcin BC. 1986. Sheep and goats in Turkey. Food and Agriculture Organization of the United Nations, Animal Production and Health, Paper N. 60, Rome, available at http://www.fao.org/docrep/009/ah224e/ah224e00.htm.