Abstract
Novel creatine bienzymatic potentiometric biosensors were prepared by immobilizing urease and creatinase on all solid-state contact PVC-containing palmitic acid and carboxylated PVC matrix membrane ammonium-selective electrodes without inner reference solution. Potentiometric characteristics of biosensors were examined in physiological model solutions at different creatine concentrations. The linear working range and long-term sensitivity of the biosensors were also determined. The creatine biosensors prepared by using the carboxylated PVC membrane electrodes showed more effective performance than those of the PVC containing palmitic acid membrane electrodes. Creatine assay in serum samples was successfully carried out by using the standard addition method.
Introduction
Creatine plays an important role in the metabolism of proteins. Therefore, it appears to increase lean body mass and strength in humans (Stefan et al. Citation2006). Creatine is an important diagnostic substance in the assay of renal, thyroid, and muscular functions, and testing its clearance could also offer a quick and relatively simple biomedical diagnosis of acute myocardial infarction. Therefore determination of creatine in biological fluids is of great interest in clinical diagnosis (Kim et al. Citation1999, Wang et al. Citation2006, Stefan et al. Citation2003, Lakshmi et al. Citation2007, Karakus et al. Citation2006). The typical human reference range for serum/plasma creatine is between 35–140 µM; it can rise to greater than 1000 µM during kidney dysfunction (Stefan et al. Citation2003, Sena et al. Citation1988, Lad et al. Citation2008, Pan et al. Citation2011).
The following techniques are proposed for the assay of creatine; HPLC (CitationSamanidou et al. 2010, CitationYang 1988), mass spectroscopy (Schwedhelm Citation2000), IR spectroscopy (Pezzanti et al. Citation2001), capillary zone electrophoresis (Clark et al. Citation2001, Burke et al. Citation1999), and flow injection analysis systems with electrochemical and spectrometric detection (Del Campo Citation1995). Among them, electrochemical enzymatic biosensors seem to be promising tools for such analysis, and various types of such biosensors for creatine detection have been developed (Stefan et al. Citation2003, Citation2006, Lakshmi et al. Citation2007, Patel et al. Citation2010, Motonaka et al. Citation1988, Koncki et al. Citation1996). Due to simplicity and low cost, potentiometric enzyme sensors are most widely applied among electrochemical enzymatic biosensors (Elmosallamy Citation2006, Soldatkin 2002, Shin et al. Citation1998, Koncki et al. Citation2000). In the literature, some of these biosensors are based on the catalysis of creatine at the surface of an ammonium-selective electrode. However, the biosensor prepared by immobilizing enzymes on ammonium-selective electrode membrane containing nonactin as ammonium ionophore and carboxylated PVC as the polymer membrane matrix material were expressed to be low sensitivities against creatine biomolecules (Koncki et al. Citation1996, Ma et al. Citation1988).
In recent years, attempts were made to develop miniaturized potentiometric biosensors, which are particularly important to reduce the amount of enzyme and reagents needed (Soldatkin et al. Citation2002, Bilitewski Citation1992). The miniaturization of a biosensor is possible by using an all-solid-state contact polymer membrane ion-selective electrode (Chou et al. Citation2009, Pookaiyaudom et al. Citation2011, Suzuki et al. Citation2001. Using the all-solid-state structure, the sensing devices provide many advantages, including simplicity of fabrication, being in the solid state form, ease of packaging, dry storage, and low cost (Alegret et al. Citation1989, Tinkilic Citation2002).
In the present work, we, for the first time, report all-solid-state contact creatine biosensors based on PVC containing palmitic acid and carboxylated PVC membrane NH4+ -selective electrodes without an inner reference solution. The all-solid-state contact creatine biosensors functioned based on the catalysis of creatine by creatinase and urease enzymes at the surface of the NH4 + -selective electrodes. The performance characteristics (e.g. pH influence, reproducibility, selectivity, stability, response time, optimum working range, etc.) of the prepared all-solid-state creatine biosensors were determined. Furthermore, we investigated whether these biosensors can be used to determine creatine level in human serum samples.
Experimental
Reagents and solutions
Creatinase (Pseudomonas sp., 8.7 U/mg, E.C.3.5.3.3), nonactin, palmitic acid, bis-(2-ethyl)hexylsebacate (DOS), poly(vinylchloride) (PVC), carboxylated poly(vinylchloride) (PVC–COOH), tetrahydrofuran (THF) were obtained from Fluka. Urease (Jack Beans, 5 U/mg, E.C.3.5.1.5) was purchased from Merck (Darmstadt, Germany). 1-Ethyl-3-(3- dimetylaminopropyl) carbodiimide (EDC) was obtained from Sigma Chem. Co. (St. Louis, USA). Epoxy resin (Macroplast Su 2227) and hardener (Desmodur RFE), used in the preparation of conductive all solid contact, were purchased from Henkel (Istanbul, Turkey) and Bayer AG (Darmstadt, Germany), respectively. All of the reagents used were analytical reagent grade and deionized water was used throughout.
Apparatus
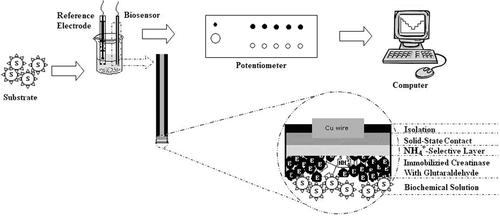
Potentiometric measurements were carried out at room temperature (20 ± 1°C) by using a laboratory-made, computer-controlled, high-input impedance, multi-channel potentiometric measurement system. Throughout the measurements, a micro-sized solid silver/silver chloride electrode was used as reference electrode (RE) with the ammonium-selective electrode and creatine biosensor. The reference electrode was obtained from Isedo Med. Ltd. Sti. (Samsun, Turkey). To investigate the potentiometric characteristics of the prepared biosensor, the emf measurements were taken in the following cell assembly:
Micro sized solid silver/silver chloride RE | test solution | Creatine biosensor membrane/Ammonium-selective electrode | all solid state contact material | Cu wire.
The steady-state measurements were taken while stirring at a constant rate by immersing the biosensor and reference electrodes to the same depth in 10 mL of tested solution. The reference electrode and biosensor were washed with deionized water and dried with adsorbent tissue before each measurement of the test solution.
The pH of the buffer solutions was adjusted by using a glass pH electrode (Russell) with a Jenway 3040 model Ion Analyser. Solutions at required concentrations were homogenized using a Ultrasonic LC30 (Germany) stirrer.
Preparation of all-solid-state ammonium-selective membrane electrode
The membrane compositions of ammonium-selective electrodes prepared by using PVC containing palmitic acid and carboxylated PVC are shown in . Each membrane, according to the compositions in , was prepared by dissolving the components in 3 mL of THF. The construction of all-solid-state contact PVC containing palmitic acid and carboxylated PVC matrix membrane ammonium selective electrodes without an inner reference solution was carried out as described below. The epoxy resin mixture used to bind the graphite in preparing the all-solid-state contact was made from epoxy and hardener in THF in the proportions 1.0:0.5 w/w. The powdered graphite was mixed with the epoxy resin mixture in the proportions 1.0:1.0 w/w. After mixing, the solution was allowed to stand for 5–10 min in air. When the appropriate viscosity was attained, a shielded copper wire end with the length of 5 cm and radius of 0.5 mm was dipped into the mixture several times to obtain a coating thickness of about 0.3 mm. The uniformly coated copper wire end was allowed to stand overnight in an oven at 40°C. The all-solid-state contact was dipped into each membrane solution (given in ) at least three times to obtain a membrane thickness of about 0.2 mm (see ). The coated membrane was left to be dried at laboratory conditions for at least 5 h. Finally, the dried all-solid-state contact ammonium-selective membrane electrode was conditioned by soaking into 0.1 mol/L ammonium chloride solution for at least 6 hours before use. When not in use, all the electrodes were stored in laboratory conditions. Before the individual measurement process, the electrodes were reconditioned for at least 10 minutes in 0.1 mol/L ammonium chloride solution. The potentiometric performance characteristics of the electrodes were tested in steady-state conditions.
Table 1. The membrane compositions of the all-solid-state contact ammonium-selective electrodes.
Preparation of creatine biosensor
Enzymes were chemically immobilized on the all-solid-state ammonium-selective membrane electrode surfaces by using three different steps as described in procedures given by Koncki et al. (Citation1994) and Karakus et al. (Citation2005, Citation2006).
(A) 4 mg creatinase, 6 mg urease, and 1 mg 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide were dissolved in 0.2 mL deionized water. 10 μl of this solution was dropped onto the membranes of ammonium-selective electrodes and left overnight (biosensor A).
(B) 10 μl of 2.5% glutaraldehyde solution was deposited on the surface of biosensor A and the biosensor was left for 0.5 h. Then the surface was washed with deionized water to remove the excess of glutaraldehyde (biosensor B).
(C) 6 mg urease and 4 mg creatinase were dissolved in 0.2 mL deionized water. 10 μl of this solution was deposited on the biosensor B surface. To remove the excess of unbounded enzymes all types of biosensors were left in the vigorously stirred phosphate buffer (pH 7.0, 10 mM) for 1 h. When not in use, biosensors were stored in a refrigerator at 4°C. The schematic diagram of all-solid-state contact PVC membrane ammonium-selective electrode with immobilized enzyme layer and the set-up of the measurement system are illustrated in .
Potential measurements for creatine biosensors were carried out by varying creatine concentration in steady-state condition. Ten millimolar of TRIS buffer was used as a working buffer solution (pH 7.0). The calibration curves were obtained by plotting the potential values of a series of standard creatine solutions against the logarithm of creatine concentration.
Procedure for determination of creatine in serum
The creatine levels in the serum samples in a concentration range of 0.97–6.81 mg/dL were succesfully determined by using the standard addition method (Premanode and Toumazou, Citation2007. The creatine biosensor was immersed in 10 mM TRIS buffer solution (at pH 7.0) containing a certain amount of serum creatine. To check our results obtained from the proposed biosensor method, the Jaffe (Citation1886) method was also used for determining creatine levels in serum samples.
Results and Discussion
Potentiometric performances of the all-solid-state ammonium-selective membrane electrodes
The potentiometric performance characteristics of the all-solid-state contact ammonium-selective membrane electrodes are summarized in . Both electrodes based on PVC containing palmitic acid and carboxylated PVC exhibited almost 47 ± 5 and 50 ± 5 mV slopes between 1 × 10–1 and 1 × 10–5 M concentration levels of ammonium, respectively. The detection limits of both electrodes were about 2 × 10–6 and 3 × 10–6 M and the response times of the electrodes were less than 15 and 10 s, respectively. The ion-selective membrane electrodes with slopes in that vicinity range value and sensitivity could be used for analytical purposes such as biosensor development (Karakus et al. Citation2006, Buck Citation1972, Wegmann et al. Citation1984, Evans Citation1991).
Table 2. Potentiometric performance characteristics of the all-solid-state contact ammonium-selective membrane electrodes.
Although the sensitivity and the response time of both electrodes were comparable, the slope of the ammonium-selective electrode prepared by using carboxylated PVC (membrane II) was a bit better than that of the electrode prepared by using PVC containing palmitic acid (membrane I), as shown in . However, in respect to overall potentiometric performance, the present results obtained with the ammonium-selective electrode prepared by using carboxylated PVC can be comparable and more useful than those of the ammonium selective electrodes employed in other studies (Koncki et al. Citation1996, Chou et al. Citation2009).
Potentiometric performances of all-solid-state contact creatine biosensors
As previously mentioned, the measurements with the creatine biosensors based on detection of ammonium ions was performed in 10 mM Tris buffer solution. The response times of the creatine biosensors were measured to observe the reaction of the enzymes immobilized on different electrode membranes. According to IUPAC recommendations (Richard and Erno Citation1994), t95 (95% of the steady-state signal) values of the creatine biosensors based on the all-solid-state PVC-COOH and PVC containing palmitic acid membrane ammonium-selective electrodes were less than 35 and 60 s, respectively. The response times of the prepared biosensors were also found to be better than most similar biosensors reported in the literature (Karakus et al. Citation2006, Koncki et al. Citation1996, Walcerz et al. Citation1996, CitationRadomska et al. 2006, Magalhães et al. Citation2002).
The detection limits of the creatine biosensors based on all-solid-state PVC-COOH and PVC containing palmitic acid membrane electrodes were about 30 μM with a sensitivity of 50 ± 0.8 mV/perdecade and 20 μM with a sensitivity of 35 ± 0.3 mV/perdecade, respectively. Moreover, the linear working range of both of the creatine biosensors was between 0.1–10 mM. From the obtained results it can be concluded that the creatine biosensor prepared by using carboxylated PVC exposed a wider linear working range and better sensitivity when compared to the creatine biosensor prepared by using PVC containing palmitic acid.
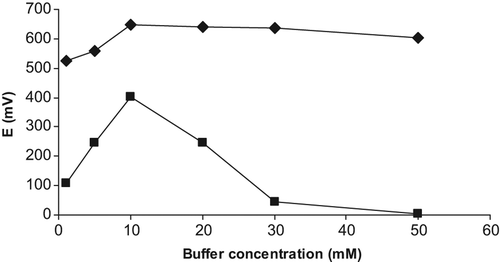
Effect of buffer concentration
Measurements of the creatine biosensors, based on detection of ammonium ions, were performed at different TRIS buffer concentrations varying from 0.1 to 50 mM. Most effective potentiometric responses of the creatine biosensors were somehow obtained at constant 10 mM creatine levels in TRIS buffer at pH 7.0 is given in .
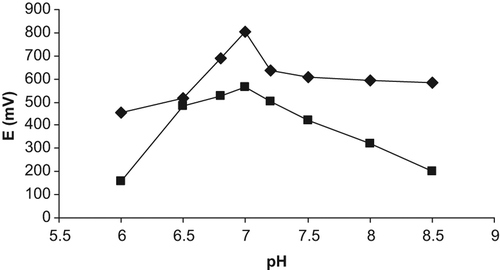
Effect of pH
Effect of pH on the analytical signal of the creatine biosensors was investigated by measurements in 10 mM TRIS buffer at different pHs (6.0–8.5). The potentiometric responses versus pH are shown in . As can be seen from , the output signals of creatine biosensors increase up to pH 7.0 and decrease over pH 7.0. This can be due to reduced activities of urease and creatinase enzymes; those might be gradually denaturized at the higher and lower pH values rather than the pH of 7.0 (Adeloju et al. Citation1996, Lucio et al. Citation1999).
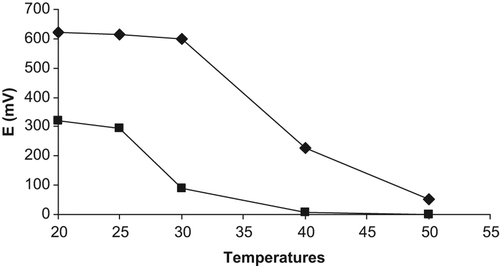
Effect of temperature
shows the effect of temperature on the response of all-solid-state contact creatine biosensors. As can be seen from , the biosensor’s responses remain almost constant between 20–25 and 20–30°C for both biosensors based on PVC containing palmitic acid and carboxylated PVC membrane ammonium-selective electrodes, respectively. The responses then decrease at higher temperatures for both biosensors because of the decrease in the activity of urease and creatinase at higher temperatures.
Selectivities of the biosensors
To simulate the application of the creatine biosensor to a real sample, the selectivity of the biosensor membrane to entities other than ammonium ion has to be considered. Therefore, the interference effects from potassium, sodium, calcium, and iron (III) ions with ascorbic acid, uric acid, and creatinine were studied. Their selectivity coefficients were evaluated by the separate solution method according to the IUPAC recommendation (Umezawa et al. Citation2000). As indicated in , interference effects of Na+, K +, Ca2 +, Fe3+ ions, ascorbic acid, uric acid, and creatinine on the response of the creatine biosensors were very low. On the other hand, as the all-solid-state contact ammonium-selective electrode solely exhibited just around 20 times higher selectivity toward ammonium ion over potassium ion. Therefore, the potassium ion was the most important interfering specie for the nonactin ionophore-membrane-based biosensors (Chou et al. Citation2009). The obtained results indicate a better selectivity than those of other potentiometric biosensors (Karakus et al. Citation2006, Walcerz et al. Citation1996). Ascorbic acid, uric acid, and creatinine that existed in serum could not expose the interference effect on the developed potentiometric creatine biosensors.
Table 3. The selectivity coefficients of creatine biosensors based on the all-solid-state contact ammonium-selective membrane electrodes
Operational and storage stability of the creatine bioensors
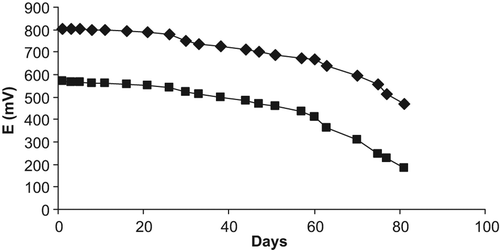
Operational and storage stability of the creatine biosensors were determined by reading the potential values of the calibration solutions and by plotting the calibration curves for a period of 80 days (). Studies corresponding to storage stability indicate that the biosensor did not lose any activity for almost 30 days. After 30 days, the biosensor response retained about 32 and 58% of the initial activity of the creatine biosensors based on a PVC containing palmitic acid and carboxylated PVC membrane electrodes, respectively. The decrease in response could be attributed to the denaturizing of the surface of immobilized enzyme (Chou et al. Citation2009). The responses were also almost constant during 12 times at operation at 10 mM TRIS buffer, pH 7.0-. The relative standard deviations of the biosensor responses were found to be less than 1,2 % (n:12) for both PVC containing palmitic acid and carboxylated PVC membrane electrodes. These results were better than those of amperometric and potentiometric creatine biosensors in some other studies (Stefan et al. Citation2006, Citation2003, Karakus et al. Citation2006, Koncki et al. Citation1996).
Application to creatine determination in human serum
The all-solid-state contact creatine biosensor based on carboxylated PVC was applied to human serum samples for the determination of creatine. Creatine levels in human serum samples obtained with the biosensor method by the standard addition method were also correlated with the Jaffe method (Koncki et al. Citation2000). The obtained result is given in . As indicated in , good correlation between the results was obtained using both the Jaffe method and proposed creatine biosensor method. The obtained results prove the usefulness of the biosensor for determination of creatine in serum samples.
Table 4. Determination of creatine in serum samples (R.S.D. values are lower than 0.1%).
Conclusions
The novel all-solid-state contact creatine biosensor based on a carboxylated PVC ammonium-selective electrode showed good analytical parameters such as high selectivity and sensitivity (20 μM), storage stability over 30 days with less decrease of sensitivity, and short response time (0.5 min). Some other useful properties of the biosensor developed here are low cost, ease of preparation and use, and good operational stability in static conditions. The all-solid-state creatine biosensor can easily be miniaturized and applied to direct creatine detection in human serum. Ascorbic acid, uric acid, and creatinine did not exhibit interference effect on the response of the proposed potentiometric creatine biosensor. This is an advantage for creatine determination in serum samples when it is compared with other amperometric biosensors (Stefan et al. Citation2003, Citation2006, Lakshmi et al. Citation2007).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adeloju SB, Shaw SJ,Wallace GG. 1996. Polypyrrole-based amperometric flow injection biosensor for urea. Anal Chim Acta 323:107–13.
- Alegret S, Martinez-Fabregas E. 1989. Biosensors based on conducting filled polymer all-solid state PVC-matrix membrane electrodes. Biosensors 4:287–297.
- Bilitewski U, Chemnitius GC, Ruger P, and Schmid RD. 1992. Miniaturized disposable biosensors. Sensors and Actuators B 7:351–355.
- Buck RP. 1972. Ion selective electrodes, potentiometry and potentiometric titrations. Anal Chem 44:270R.
- Burke DG, MacLean PG, Walker RA, Dewar PJ, Palmer TS. 1999. Analysis of creatine and creatinine in urine by capillary electrophoresis. J Chromatogr B 732:479–485.
- Chou NH, Chou JC, Sun TP, Hsiung SK. 2009. All solid-state potentiometric biosensors for creatinine determination based on pH and ammonium electrodes. IEEE Sensors Journal 9:665–672.
- Clark EA, Fanguy JC, Henry CS. 2001. High throughput multi-analyte screening for renal disease using capillary electrophoresis. J Pharm Biomed Anal. 25:795–801.
- Del Campo, G., Irastorza, A., Casado, J.A. 1995. Spectrophotometric simultaneous determination of creatinine and creatine by flow injection with reagent injection. Fresenius J Ana. Chem 352:557–561.
- Elmosallamy MAF. 2006. New potentiometric sensors for creatinine. Analytica Chimica Acta 564:253–257.
- Evans A. 1991. Potentiometry and ion-selective electrodes, Wiley, New York.
- Jaffe M. 1886. Uber den niederschlag welchen pikrinesaure in normalen harn ergeugt and ubereine neue reaktion des kreatinins: Hoppe-Seyler's. Z Physiol Chem 10:391–400.
- Karakus E, Pekyardımcı S, Kılıc E. 2005. Urea biosensors based on PVC membrane containing palmitic acid. Artif Cells Blood Substit Immobil Biotechnol 33(3):329–41.
- Karakus E, Pekyardımcı S, Kılıc E. 2006. Potentiometric bienzymatic biosensor based on PVC membrane containing palmitic acid for determination of creatine. Process Biochemistry 41:1371–1377.
- Kim EJ, Haruyama T, Yanagida Y, Kobatake E, Aizawa M. 1999. Disposable creatinine sensor based on thick-film hydrogen peroxide electrode system. Anal Chim Acta 394:225–31.
- Koncki R, Kopczewska E, Glab S. 1994. Comparison of pH-membrane enzyme electrode for urea with covalently bound enzyme. Anal Lett 27(3):475–86.
- Koncki R, Radomska A, Glab S. 2000. Bioanalytical flow-injection system for control of hemodialysis adequacy. Anal Chim Acta 418:213–224.
- Koncki R, Walcerz I, Ruckruh F, Glab S. 1996. Bienzymatic potentiometric electrodes for creatine and L-arginine determination. Anal Chim Acta 333:215–22.
- Lad U, Khokhar S, Kale GM. 2008. Electrochemical creatinine biosensors. Anal Chem 80:7910–7917.
- Lakshmi D, Sharma PS, Prasad BB. 2007. Imprinted polymer-modified hanging mercury drop electrode for differential pulse cathodic stripping voltammetric analysis of creatine. Biosensors and Bioelectronics 22:3302–3308.
- Lucio D, Daniela F, Silvano L, Giancarlo P. 1999. Amperometric biosensor involving covalent immobilization of choline oxidase and butyrylcholinesterase on a methacrylate-vinylene carbonate co-polymer. Biotechnol Appl Biochem 29:67–72.
- Ma SC, Chaniotakis NA, and Meyerhoff ME. 1988. Response properties of ion-selective polymeric membrane electrodes prepared with aminated and carboxylated poly(vinyl chloride). Anal Chem 60:2293–2299.
- Magalhães MCS, Machado AASC. 2002. Array of potentiometric sensors for the analysis of creatinine in urine samples. The Analyst 127:1069–1075.
- Motonaka J, Takabayashı SI, Tanaka N. 1988. The preparation and properties of an enzyme electrode for creatine. Bull Chem Soc Jpn 61:3342–3343.
- Pan TM, Lin CW, Lin WY, Wu MH. 2011. High-κ Tm2Ti2O7 electrolyte-insulator-semiconductor creatinine biosensor. IEEE Sensors Journal, 11:2388–2394.
- Patel AK, Sharma PS, Prasad BB. 2010. Trace-level sensing of creatine in real sample using a zwitterionic molecularly imprinted polymer brush grafted to sol–gel modified graphite electrode. Thin Solid Films 518:2847–2853.
- Pezzanti JL, Jeng TW, McDowell L, Oosta GM. 2001. Preliminary investigation of near-infrared spectroscopic measurements of urea, creatinine, glucose, protein, and ketone in urine. Clin Biomed 34:239–246.
- Pookaiyaudom P, Seelanan P, Lidgey FC, Hayatleh K, Toumazou K. 2011. Measurement of urea, creatinine and urea to creatinine ratio using enzyme based chemical current conveyor (CCCII +). Sensors and Actuators B 153:453–459.
- Premanode B, Toumazou C. 2007. A novel, low power biosensor for real time monitoring of creatinine and urea in peritoneal dialysis. Sensors and Actuators B 120:732–735.
- Radomska A, Koncki R, Pyrzynska K, Glab S. 2004. Bioanalytical system for control of hemodialysis treatment based on potentiometric biosensors for urea and creatinine. Analytica Chimica Acta 523:193–200.
- Richard PB, Erno L. 1994. Recommendations for nomenclature of ionselective electrodes. Pure and Appl Chem 66:2527–2536.
- Samanidou VF, Metaxa AS, Papadoyannis IN. 2002. Direct simultaneous determination of uremic toxins: creatine. creatinine, uric acid, and xanthine in human biofluids by HPLC. J. Liq Chromatogr Relat Technol 25:43–57.
- Schwedhelm E, Tsikas D, Durand T, Gutzki FM, Guy A, Rossi JC, Froelich JC. 2000. Tandem mass spectrometric quanti_cation of 8-iso-prostaglandin F2_ and its metabolite 2, 3-dinor-5,6-dihydro-8-iso-prostaglandin F2_ in human urine. J Chromatogr B: Biomed Appl 744:99–112.
- Sena SF, Syed D, McComb RB. 1988. Effect of high creatine content on the Kodak single-slide method for creatinine. Clin Chem 134:594–595.
- Shin JH, Yoon SY, Yoon IJ, Choi SH, Lee SD, Nam H, Cha GS. 1998. Potentiometric biosensors using immobilized enzyme layers mixed with hydrophilic polyurethane. Sensors and Actuators B 50:19–26.
- Soldatkin AP, Montoriol J, Sant W, Martelet C, Jaffrezic-Renault N. 2002. Development of potentiometric creatinine-sensitive biosensor based on ISFET and creatinine deiminase immobilised in PVA/SbQ photopolymeric membrane. Materials Science and Engineering C 21:75–79.
- Stefan RI, Bokretsion R.G, van Staden JF, Aboul-Enein HY. 2003. Simultaneous determination of creatine and creatinine using amperometric biosensors Aboul-Enein Talanta 60:1223–1228.
- Stefan RI, Bokretsion RG, van Staden JF, Aboul-Enein HY. 2006. Simultaneous detection of creatine and creatinine using a sequential ınjection analysis/biosensor system preparative. Biochemistry & Biotechnology 36:287–296.
- Suzuki H, Arakawa H, Karube I. 2001. Fabrication of a sensing module using micromachined biosensors. Biosensors & Bioelectronics 16:725–733.
- Tinkilic N, Cubuk O, Isildak I. 2002. Glucose and urea biosensors based on all solid-state PVC-NH2 membrane electrodes. Anal Chim Acta 452:24–29.
- Umezawa Y, Buhlmann P, Umezawa K, Tohda K, Amemiya S. 2000. Potentiometric selectivity coefficients of ion-selective electrodes. Pure Appl Chem 72(10):1851–2082.
- Walcerz I, Koncki R, Leszczynska E, Salamonowicz B, Glab S. 1996. Enzyme biosensors for urea determination based on an ionophore free membrane electrode. Anal Lett 29(11):1939–53.
- Wang Y, Ma X, Zhao W, Jia X, Kai L, Xu X. 2006. Study on the creatinase from Paracoccus sp. strain WB1 Process Biochemistry 41:2072–2077.
- Wegmann R,Weiss H, Ammann D, Morf WE, Pretsch E, Sagahara K, . 1984. Anion selective liquid membrane electrodes based on lipophilic quaternary ammonium compounds. Microchim cta 111:1–6.
- Yang YD. 1998. Simultaneous determination of creatine: Uric acid, creatinine and huppuric acid in urine by HPLC. Biomed. Chromatogr 12:47–49.