Abstract
A series of gelatin hydrogels were prepared by crosslinking method using glutaraldehyde (GA). The hydrogels were characterized by gel formation, swelling/degradation tests, and FTIR analysis. The variations of swelling percentages (S%) with time, temperature, and pH were determined. It is found that the increasing amount of GA causes the decreasing in S% values from 366 to 213% and G-1 was found to be the most swollen hydrogel at pH 7.4 and 37°C. Degradation tests of hydrogel samples were carried out and G-1 hydrogel, which contained the least amount of GA, degraded more rapidly than the others. G-2 hydrogel was chosen for immobilization studies and this procedure was carried out by activation of the hydrogel disc with N-(3-dimethylaminopropyl)-N-ethylcarbodiimid (CDI) coupling agent. The kinetic parameters, Km and Vmax, were calculated. Km values of free and immobilized lipases were found to be 0.290, 0.422 mM while Vmax values were calculated as 0.089, 0.080 mM.min−1, respectively. For the free and immobilized system, the enzymes retained 32% and 92% of their initial activities, respectively, at the end of 48 days of storage. After using the mentioned immobilized system repeatedly 10 times, it retained 68% of its original activities.
Introduction
Hydrogels are hydrophilic three-dimensional networks held together by crosslinked chemical or physical bonds. They are commonly used in clinical practice and experimental medicine for a wide range of applications, including tissue engineering and regenerative medicine, diagnostics, cellular immobilization, separation of biomolecules or cells, and barrier materials to regulate biological adhesions. Hydrogels are also generally highly biocompatible, as reflected in their successful use in vivo. Biodegradability or dissolution may be designed into hydrogels via enzymatic, hydrolytic, or environmental (e.g. pH, temperature, or electric field) pathways. Hydrogels are also relatively deformable and it can conform to the shape of the surface to which they are applied (Hoare and Kohane 2008, Chena et al. 2003, Sakai et al. 2009).
Among other biopolymers, gelatin has been extensively studied due to its low cost, biodegradability, biocompatibility, and non-immunogenic properties. It is widely used in the pharmaceutical industry as well as in the biomedical field: hard and soft capsules, microspheres, sealants for vascular prostheses, wound dressing and adsorbent pad for surgical use are among its most frequent applications (Sakai et al. 2009, Sokker et al. 2009, Young et al. 2005). Gelatin is obtained by thermal denaturation or physical and chemical degradation of collagen; the most widespread protein in the body occurs in most connective tissues as skin, tendon, and bone (Kushibiki et al. 2003, Gilsenan and Ross-Murphy 2001).
Gelatin exhibits poor mechanical properties, which limit its possible applications as a biomaterial. On the other hand, it readily undergoes chemical crosslinking because of the large number of functional side groups. Mechanical and thermal stability of gelatin materials are improved by crosslinking method. Chemical crosslinking agents, such as formaldehyde, glyoxal, glutaraldehyde (GA), genipin, and transglutaminase, are used to modify gelatin samples. GA is an amine-reactive homobifunctional reagent. It is frequently used to prepare the crosslinked hydrogels and covalently bonded enzyme molecules. The materials crosslinked with GA can be stored for a long time. GA has undoubtedly found the widest application in various fields such as histochemistry, biomedical, pharmaceutical sciences, microscopy, leather tanning industry, enzyme technology, and chemical sterilization (Cortesi et al. 1998, Migneault 2004).
As biocatalysts, enzymes exhibit a number of advantages such as high level of catalytic efficiency and high degree of selectivity. However, there exist various practical problems in the enzyme applications; for example, high cost and instability. Thus, enzymes are often immobilized onto solid supports to increase their operational stability and recoverability. Immobilization methods can be divided into two general classes: chemical and physical methods. Covalent bonds are formed with the enzyme in chemical methods. Weak interactions existed between support and enzyme in physical methods. Covalent enzyme immobilizations often begin with a surface modification or activation step. Generally, the carbodimides coupling agent is used for activation (Chiou and Wen-Tang 2004, Altun and Cetinus 2007). Enzymes have a wide variety of biotechnological, biomedical, and pharmaceutical applications. Specifically, lipases, which catalyze the hydrolysis of esters such as glyceride, are industrially useful enzymes, finding wide use in oil processing, production of surfactants, and preparation of enantioselective pharmaceutics. For their industrial applications, immobilized lipases have been specifically studied due to their enhanced stability, easy separation, and reusability. Various immobilization methods and supports have been developed in order to improve their activity (Balcao et al. 1996, Ting et al. 2006, Nasratun et al. 2008).
The main purpose of this study is to develop a lipase- immobilized gelatin hydrogel system. This system is expected to be suitable for potential use in biological systems as gelatin is a biocompatible and non-immunogenic polymer. We planned to choose the suitable hydrogel for immobilization studies depending on swelling/degradation behaviors. The covalent binding method was used to prepare immobilization of lipase onto the gelatin hydrogel. It was also designed to investigate the activity, kinetic parameters, storage stabilities, and reusability capacities of lipase-immobilized gelatin hydrogel.
Methods
Materials
Gelatin (Type B, 280 Bloom, from pig skin) was obtained from Fluka. Lipase (from Candida rugosa), p-nitrophenyl palmitate (p-NPP), N-(3-dimethylaminopropyl)-N-ethylcarbodiimid (CDI), GA (25% aqueous solution), ethanol, and phosphate buffer (PB) tablets at different pH values were purchased from Sigma-Aldrich Chemical Corporation. KBr (IR grade) and NaOH were obtained from Merck, Germany. Britton-Robinson buffer (BRB) solutions were prepared as given in the literature (Britton and Robinson 1931).
Preparation of hydrogels
In this study, four types of gelatin hydrogels were prepared by mixing 10% of aqueous gelatin solution with different amounts of GA given in . Crosslinking reaction was pursued for 24 h at room temperature in a glass tube. Glass tubes were broken and cylinder-shaped fresh hydrogel rods were cut into pieces 0.5 cm long.
Table 1. The amount of the components used to form the hydrogels and the gel formation percentages.
Hydrogel discs were left overnight at room conditions and washed several times with distilled water to remove unreacted chemicals. The samples were dried first in air and then in a vacuum oven at 37°C and were stored for further use later. The dimensions of the dried hydrogels were measured with a micrometer. The average thicknesses were 0.35 ± 0.05 cm, with the radius differing between 0.40 and 0.50 cm according to the content of the gel matrix.
The gel formation percentages of the samples were gravimetrically determined as follows (Chen et al. 2005).
The prepared hydrogel discs were dried, weighed, and then placed in distilled water for 48 h to extract the unreacted monomers. The hydrogels were then taken out from the extraction medium and dried in a vacuum oven at 40°C to constant weight. The gel formations (%) were determined using the following formula:
where m is the weight of the dried hydrogel after extraction and m0 is the weight of the dried hydrogel before extraction. All measurements were performed in triplicate.
FTIR measurements
FTIR spectra of gelatin, CDI-activated gelatin hydrogel, lipase, lipase-immobilized gelatin hydrogel were recorded using a Perkin-Elmer 1710 model spectrophotometer.
About 1 mg of the samples were pounded and completely mixed with KBr and pellets were prepared using a hydraulic press under a pressure of 600 kg/cm2. Spectra were scanned between 4000 and 400 cm−1.
Swelling studies
Swelling tests of hydrogel discs were gravimetrically carried out in three steps. In the first step, dried discs were left to swell in a PBS (pH 7.4) at 37°C. Swollen gels were removed from the swelling medium at regular intervals and dried superficially with filter paper, weighed, and placed into the same bath. The measurements were continued until a constant weight reached for each sample. The percentage swelling (S%) values were calculated from the following equation (Chen et al. 2005, Hsiue et al. 2001):
where mw is the wet weight of the sample and md is the dry weight of the sample before swelling. The incubation times for all gels were approximately 48 h.
In the second step, the dried hydrogel discs were swollen in PBS (pH 7.4) solutions at different temperatures ranging from 4 to 60°C so that we could investigate the effect of temperature on swelling behaviors. At the end of 48 h, the swollen discs were removed from the swelling medium, dried superficially with filter paper, and weighed. S% values were calculated with Equation (2). In the last step, the same procedure was performed in different BRB solutions at various pHs between 2 and 12 so that we could investigate the effect of pH on the swelling behaviors. The temperature and swelling time were kept constant (37°C and 48 h, respectively). The reproducible results for all swelling studies were obtained with triplicate measurements.
Degradation test
Degradation tests of hydrogel samples were carried out at pH 7.4 and 37°C (Chen et al. 2005, Tan et al. 2009). Dried hydrogel discs were left to swell in PBS. Swollen gels were removed from the swelling bath at the end of 48 h, dried superficially with filter paper, and weighed. This mass (mm) was recorded as the maximum swollen state of hydrogels. Then they were placed into the same bath and weighing was continued at regular intervals until the hydrogels completely degraded. The degradations (%) were determined using the following formula:
where mm is the weight of hydrogel at the most swollen stage and mt is the weight of hydrogel at time t. All measurements were performed in triplicate.
Immobilization of lipase on gelatin hydrogel
Depending on swelling and degradation test results, G-2 hydrogel was chosen for lipase immobilization. First, the functional groups of gelatin were activated with CDI coupling agent (Chiou and Wen-Tang 2004, Hung et al. 2003). The dried disc was left to swell in 25.0 mL of PBS (pH 6.9) at 24 h and then it was put into 20.0 mL of %1 (w/v) CDI solution (pH 6.9) at 25°C. The system was shaken for 4 h and then left overnight at room temperature. Gelatin discs were removed from activation medium, and washed several times with PBS. An activated disc was put into 25.0 mL of lipase solution (0.4 mg/mL) and the immobilization process was performed at 25°C for 2 h. After this period, the mixture was left for 24 h at 4°C. Finally, the immobilized disc was taken out from the medium, dried in air conditions, and then stored at 4°C for further use later (Xiao-Jun et al. 2007). The amount of unbound enzyme in immobilization solution was determined by spectrophotometric measurements at 305 nm.
Activity test of free and immobilized lipase and kinetic study
The activity of free and immobilized lipases was determined via the hydrolysis reaction of p-NPP (Xiao-Jun et al. 2007, Gupta et al. 2002). One unit (U) of lipase activity was defined as the amount of enzyme necessary to hydrolyze 1μmol of p-NPP per min (20). 5.0 mL of PBS (pH 7.4), 5.0 mL of 1.3 mM p-NPP and 1.0 mL of 0.4 mg/mL free lipase solutions (or 1.0 mg immobilized lipase per 50 mg gelatin disc) were mixed and left at 37°C. After 5 min, absorbance of supernatant was spectrophotometrically measured at 275 nm. The decrease in absorbance is caused by hydrolysis of p-NPP. To compare the free and immobilized lipase activities, initial rate (V0) values were calculated from Equation (4) (Marangoni 2003):
where ∆c is the variation of p-NPP concentration and ∆t is reaction time.
Two important kinetic parameters for enzymes are Km and Vmax.. Vmax defines the maximum rate obtained for an enzyme in the presence of excess substrate. Km, the Michaels constant, defines the concentration of substrate at which the rate of reaction is equal to one half of Vmax (Holum 1968). Km and Vmax values of free and immobilized lipases are determined from a Lineweaver–Burk plot by measuring V0 at different substrate concentrations. In this study, substrate concentrations [S] were varied from 0.25 to 2.00 mM at 37°C (pH 7.4). The Lineweaver-Burk equation is given in Equation (5) (Marangoni 2003):
1/V0 was plotted against 1/[S]; a straight line was obtained with a slope of Km/Vmax and an intercept of 1/Vmax.
Storage stability
One of the most important parameters to be considered in enzyme immobilization is storage stability. In this study, storage stabilities of free and immobilized lipases stored at 4°C were based on V0 values. Experiments were performed according to the procedure described previously at regular intervals in 48 days and V0 values calculated from Equation (4). Storage stability results were given as relative activity. Relative activity at each data point was calculated from the ratio of residual activity to initial activity. Initial activity was defined as 100% and residual activity was normalized to the initial activity determined at 37°C, pH 7.4 for 48 days (Dai et al. 2010).
Reuse stability of immobilized lipase
The prominent characteristic of an immobilized enzyme is its reusability over an extended period of time (Dai et al. 2010). Thus, reuse stability studies are very important for immobilized enzymes. The conditions used to measure the reusability were the same as those used for the storage stability assay. Experiments were repeated 10 times in one day. Reuse stability values were calculated from the values of initial and residual activity. Results were given as relative activity. The residual activity was normalized to the initial activity determined at 37°C, pH 7.4. The initial activity was defined as 100%.
Results
Gel formation
Gel formation percentages of the hydrogels calculated via Equation (1) are given in . In general, high gel formation values were mostly obtained.
Swelling behaviors of the hydrogels
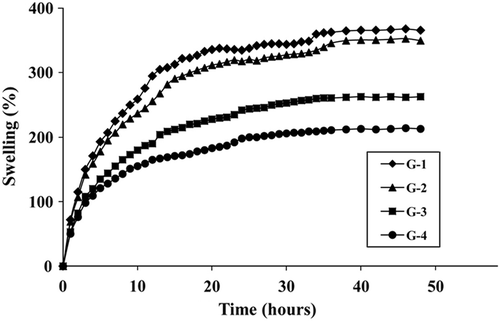
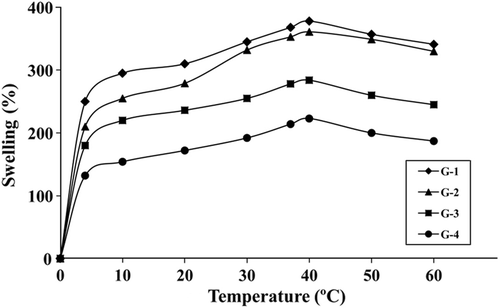
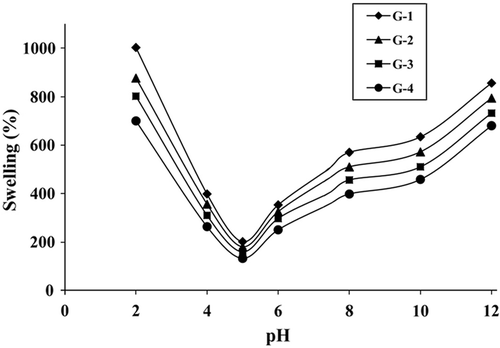
represents the variation of S% values with time at pH 7.4 and 37°C. S% increased with time initially and then remained constant at close to 48 h. S% values were determined to be 368% for the most swollen hydrogel G-1, and 213% for the least swollen hydrogel G-4. presents the variation of S% values of hydrogels with temperature at pH 7.4 and 48 h. All hydrogels slightly swelled more at high temperatures than at low temperatures. shows the variation of S% values with pH at 37°C and 48 h. In all compositions, the maximum extent of swelling was reached at pH 2.0. S% values first decreased significantly as pH increased, reached a minimum, and then increased. The minimum S% values were obtained for all hydrogels at pH 5.0.
Degradation behaviors of hydrogels
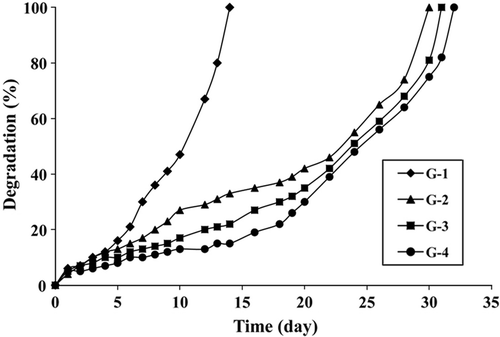
Gelatin is a natural and biodegradable polymer. As gelatin hydrogel/lipase systems prepared in this study are planned to be potentially used in vivo systems, degradation behaviors of hydrogels are very important. shows degradation behaviors of gelatin hydrogels at 37°C and pH 7.4. The fastest degradation was observed for the G-1 hydrogel, which completely degraded in 14 days. Other hydrogels showed similar behaviors and they degraded in approximately 30–32 days.
FTIR spectra
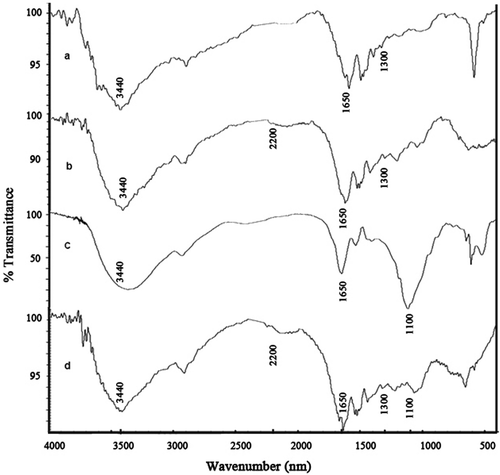
The FTIR spectra of pure gelatin, CDI-activated gelatin hydrogel, pure lipase, and lipase-immobilized gelatin hydrogel are shown in .
Activities of free and immobilized lipases
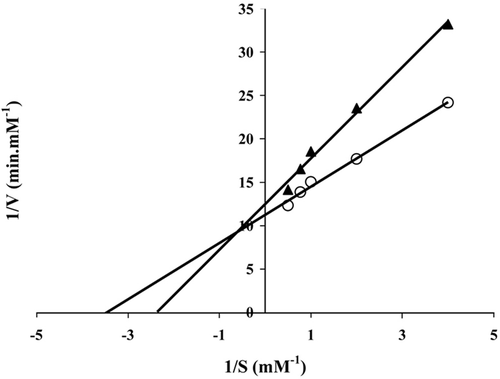
Depending on gel formation and swelling/degradation results, the G-2 hydrogel was chosen for lipase immobilization. G-1 hydrogel was not convenient because of its rapid degradation. G-3 and G-4 hydrogels weren’t preferred due to their high amounts of GA. Effect of substrate concentration was investigated and Lineweaver–Burk plots were drawn (). Km and Vmax values were calculated from these plots.
Storage and reuse stability of the immobilized lipases
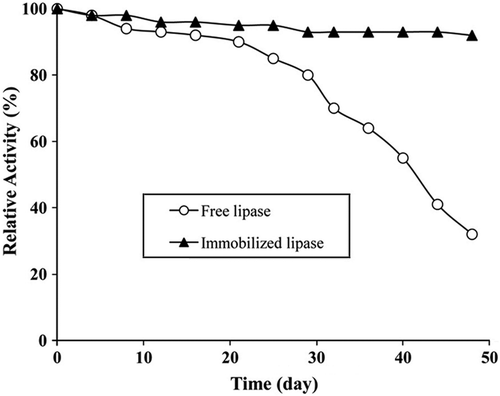
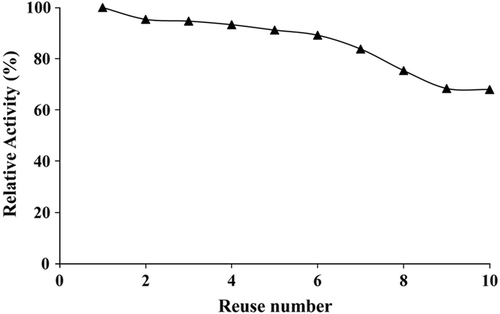
Storage stability results are given in . Immobilized lipase exhibited good performance throughout 48 days at pH 7.4. It was determined that the immobilized lipase stored at 4°C retained 92% of its original activity after 48 days, whereas the free lipase stored at 4°C retained 32% of its activity after the same period. Reuse stability results of the immobilized lipase are shown in . After 10 reuses, immobilized lipase activity decreased and retained 68% of its original activity.
Discussion
Gel formation
In general, high gel formation values were mostly obtained. By comparing the percentages, it can be thought that the increasing amount of GA positively effects gel formation. The highest and the lowest gel formation percentages were obtained for G–4 and G–1 hydrogels, respectively.
Swelling behaviors of the hydrogels
shows that S% increased with time initially and then remained constant at close to 48 h. S% values were determined to be 368% for the most swollen hydrogel G-1, and 213% for the least swollen hydrogel G-4. The results show that S% values strongly decrease with increasing GA concentration. This situation can be explained by crosslinked density. Crosslinking markedly reduces the degree of swelling. There are numerous reports in the literature that the greater crosslinker gets the intensive crosslinking (Hsuie et al. 2001, Karadag and Saraydin 2002).
indicates that all hydrogels slightly swelled at high temperatures than at low temperatures. As the temperature increases, thermal mobility of the polymer chains also increases and hydrogen bonds are broken so hydrogels can easily swell (Xiao et al. 2005, Bosch and Gielens 2003). On the other hand, significant difference in S% values between 4 and 60°C wasn't observed. Thus, it is thought that these hydrogels are not sensitive to variations in the temperature in this range.
shows that in all compositions, the maximum extent of swelling was reached at pH 2.0. S% values first decreased significantly as pH increased, reached a minimum, and then increased. The minimum S% values were obtained for all hydrogels at pH 5.0, this being due to the isoelectric point (IEP) of gelatin. IEP is the pH value at which the molecule carries no electrical charge or the negative and positive charges are equal (Smidsrod and Moe 2008). The net charge on the amphoteric molecule is affected by pH of their surrounding environment and can become more positively or negatively charged due to the loss or gain of protons. At a pH below the IEP, the amino groups of gelatin change to become positively charged (+), and above the IEP carboxylic group of gelatin changes to become more negatively charged (−). In this case, gelatin chains repel each other and hydrogels swell rapidly. As S% values of all hydrogels exhibit great differences between pH 2.0 and pH 12.0, it is thought that these hydrogels are sensitive to variations in the pH in this range.
Degradation behaviors of hydrogels
indicates that the fastest degradation was observed for the G-1 hydrogel, which completely degraded in 14 days. Other hydrogels showed similar behaviors and they degraded in approximately 30–32 days. The results indicate that the resistance to degradation increases with the crosslink density. As expected, the G-1 hydrogel synthesized with minimum amount of GA degrading more rapidly than others. The results are in agreement with those in the literature (Van Dijk-Wolthuis et al. 1997, Yang et al. 2010).
FTIR spectra
As the components consist of similar groups, the spectrums include identical absorption peaks in . In general, all of the curves show the analogous broad band attributed to gelatin and lipase at ∼ 3440 cm−1 due to N-H stretching vibrations, associated −OH stretching vibrations of the hydroxyl group, and at ∼ 1650 cm−1 belongs to C = O stretching (amide I). At curve a, belonging to pure gelatin, the absorption bands at ∼ 2920 cm−1 (the aliphatic 2CH stretching vibrations), at ∼ 1300 cm−1 (Amide III), at ∼ 1650 cm−1 (Amide I), and at ∼ 650 cm−1 (N-H out-of-plane wagging) are characteristic of its structure. At curve b, belonging to CDI-activated gelatin hydrogel, the strong band appearing at ∼ 1642 cm−1 due to C = N, C = C and C = O stretching vibrations from Schiff-base linkages in the crosslinked disc. The intense and wide absorption at ∼ 1005 cm−1 is generated by overlapping of C-O, C-N, and C-C stretching of mixed origin. The band ∼ 2200 cm−1 belongs to N = C = N stretching vibration also confirms the existence of CDI. It can be said that the hydroxyl groups of gelatin were activated with CDI. At curve c, belonging to pure lipase, the strong and intensive absorption bands at ∼ 1100 cm−1 (stretching of the C-N-C bridge) are characteristic of its lipase structure. At curve d, belonging to lipase-immobilized gelatin hydrogel, due to both gelatin and lipase having the same basic structure, we observed no significant difference in the vibration bands but the peak at ∼1100 cm−1 was observed corresponding to stretching of the C-N-C bridge (see curve c) of lipase (Williams and Fleming 1973, Nakamoto 1986).
Activities of free and immobilized lipases
From the experimental data, Km values were found to be 0.290, 0.422 mM for free and immobilized lipases, respectively. Vmax values were calculated as 0.089, 0.080 mM.min−1 for free and immobilized lipases, respectively (). There is an obvious increase in Km values for immobilized lipase. This parameter reflects the effective characteristics of the enzyme and depends upon diffusion effects. The increase in Km values is either due to the conformational changes of the enzyme, resulting in a lower possibility to form a substrate-enzyme complex or to the lower accessibility of the substrate to the active sites of the immobilized enzyme caused by the increased diffusion limitation. The increase in Km upon immobilization has been reported in literature (Tumturk et al. 2007, Yigitoglu and Temocin 2010). For free and immobilized lipases, the calculated Vmax values were found to be close to each other, but they were higher in free lipase than in immobilized lipase. These changes in the kinetic parameters are the result of the structural changes of the enzyme upon immobilization and the difficulty in diffusion of the substrate to reach the active site of the enzyme.
Storage and reuse stability of the immobilized lipase
shows that the immobilized lipase provided a significant advantage in stability over the free lipase, especially at a longer storage time. It was reported that the storage stability enhancement was one of the general advantages of the immobilized enzymes (Chiou and Wen-Teng 2004, Hung et al. 2003, Yigitoglu and Temocin 2010).
indicates that immobilized lipase activity decreased. This result is due to the inactivation of the enzyme caused by the denaturation of protein. The similar behavior of immobilized enzyme was reported in the literature (Nakane et al. 2007).
Conclusion
A series of gelatin hydrogels were prepared by using GA as a crosslinker. The variations of S% values with time, temperature, and pH were determined for these hydrogels. G-1 hydrogels prepared with a minimum amount of GA swell much more than others. It is found that the increasing amount of GA negatively affects swelling percentages of all hydrogels. In all compositions the minimum extent of S% values were reached at pH 5.0. S% increased with temperature and maximum S% was observed at 40°C for all the hydrogels. The degradation behaviors of hydrogels were also investigated and it was found that increasing amount of GA reduced the speed of degradation.
A series of activity experiments were performed for free and immobilized lipases. Although the immobilized lipase displayed a lower substrate affinity than free lipase, the immobilization improved enzyme stability for storage and reusability. It is concluded that lipase immobilized gelatin hydrogel formed in this study could be potentially developed for various biotechnological and industrial applications.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Altun G, Cetinus S. 2007. Immobilization of pepsin on chitosan beads. Food Chemistry 100(3): 964–971.
- Balcao V, Paiva A, Malcata F. 1996. Bioreactors with immobilized lipase: State of the art. Enzyme Microb. Technol 18(1): 392–416.
- Bosch E, Gielens C. 2003. Gelatin degradation at elevated temperature. International Journal of Biological Macromolecules 32: 129–138.
- Britton H, Robinson R. 1931. Universal buffer solutions and the dissociation constant of veronal. Journal of the Chem Soc 458: 1456.
- Chen K, Ku Y, Lin H, Yan T, Sheu D, Chen T, Lin F. 2005. Preparation and characterization of pH-Sensitive Poly (N-Vinyl 2-Pyrrolidone/Itaconic Acid) copolymer hydrogels. Mater Chem and Physics 91: 484–489.
- Chen L, Liu Z, Chen Y, Zhuo R. 2005. Synthesis and characterization of phosphated konjac glucomannan hydrogels. Chinese Chemical Letters 16(12): 1653–2005.
- Chena T, Embree H, Brown E, Taylor M, Paynea G. 2003. Enzyme-catalyzed gel formation of gelatin and chitosan: Potential for in situ applications. Biomaterials 24(17): 2831–2841.
- Chiou S, Wen-Teng W. 2004. Immobilization of Candida rugosa lipase on chitosan with activation of the hydroxyl groups. Biomaterials 25(2): 197–204.
- Cortesi R, Nastruzzi C, Davis S. 1998. Sugar cross-linked gelatin for controlled release: Microspheres and disks. Biomaterials 19: 1641–1649.
- Dai Y, Niu J, Liu J, Yin L, Xu J. 2010. In situ encapsulation of laccase in microfibers by emulsion electrospinning: preparation, characterization, and application. Bioresource Technology 101(23): 8942–8947.
- Gilsenan P, Ross-Murphy S. 2001. Shear creep of gelatin gels from mammalian and piscine collagens. International Journal of Biological Macromolecules 29: 53–61.
- Gupta N, Rathi P, Gupta R. 2002. Simplified para-nitrophenyl palmitate assay for lipases and esterases. Anal. Biochemistry 311: 98–99.
- Hoare T, Kohane D. 2008. Hydrogels in drug delivery: Progress and challenges. Polymer 49(8): 1993–2007.
- Holum J. 1968. Elements of General and Biological Chemistry New York: Wiley, 2nd, 377.
- Hsiue G, Guu J, Cheng C. 2001. Poly(2-hydroxyethyl methacrylate) film as a drug delivery system for pilocarpine. Biomaterials 22(13): 1763–1769.
- Hung T, Giridhar R, Chiou S, Wu W. 2003. Binary immobilization of Candida rugosa lipase on chitosan. Journal of Molecular Catalysis B: Enzymatic 26: 69–78.
- Karadag E, Saraydin D. 2002. Swelling studies of super water retainer acrylamide/crotonic Acid hydrogels crosslinked by trimetylolpropane triacrylate and 1,4-butanediol dimetacrylate. Polymer Bulletin 48: 299–307.
- Kushibiki T, Tomoshige R, Fukunaka Y, Kakemi M, Tabataa Y. 2003. In vivo release and gene expression of plasmid DNA by hydrogels of gelatin with different cationization extents. Journal of Control Release 90: 207–216.
- Marangoni A. 2003. Enzyme Kinetics, Hoboken, New Jersey: John Wiley and Sons, 44–61.
- Migneault I, Dartiguenave C, Bertrand M, Waldron K. 2004. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 37 (5): 798–802.
- Nakamoto K. 1986. Infrared and Raman Spectra of Inorganic and Coordination Compounds. Canada Polym Int: John Wiley and Sons, 234(48):228–234.
- Nakane, K, Hotta T, Ogihara T, Ogata N, Yamaguchi S. 2007. Synthesis of (Z)-3-hexen-1-yl acetate by lipase immobilized in polyvinyl alcohol nanofibers. Journal of Applied Polymer Science 106(2): 863–867.
- Nasratun M, Said H, Noraziah A, Abd Alla A. 2008. Immobilization of lipase from Candida Rugosa on chitosan beads for transesterification reaction. American Journal of Applied Sciences 6(9): 1653–1657.
- Sakai S, Hirose K, Taguchi K, Ogushi Y, Kawakami K. 2009. An injectable, in situ enzymatically gellable, gelatin derivative for drug delivery and tissue engineering. Biomaterials 30(20): 3371–3377.
- Smidsrod O, Moe S. 2008. Biopolymer chemistry. Tronheim, 43–50.
- Sokker H, Ghaffar A, Gad Y, Aly A. 2009. Synthesis and characterization of hydrogels based on grafted chitosan for the controlled drug release. Carbohydrate Polymers 75(2): 222–229.
- Tan H, Wu J, Lao L, Gao C. 2009. Gelatin/chitosan/hyaluronan scaffold integrated with PLGA microspheres for cartilage tissue engineering. Acta Biomaterialia 5(1): 328–337.
- Ting W, Tung K, Giridhar R, Wu W. 2006. Application of binary immobilized Candida rugosa lipase for hydrolysis of soybean oil. Journal of Molecular Catalysis B: Enzymatic 42(1–2): 32–38.
- Tumturk H, Karaca N, Demirel G, Sahin F. 2007. Preparation and application of poly(N,N dimethylacrylamide-co-acrylamide) and poly (N- isopropylacrylamide-co-acrylamide) /K-carrageenan hydrogels for immobilization of lipase. International Journal of Biological Macromolecules 40: 281–285.
- Van Dijk-Wolthuis W, Hoogeboom J, Van Steenbergen J, Tsang S, Hennink W. 1997. Degradation and release behavior of dextran-based hydrogels. Macromolecules 30(16): 4639–4645.
- Williams DH, Fleming I. 1973. Spectroscopic methods in organic chemistry. UK, McGraw-Hill, P. 34.
- Xiao X, Chu L, Chen W, Zhu J. 2005. Monodispersed thermoresponsive hydrogel microspheres with a volume phase transition driven by hydrogen bonding. Polymer 46(9): 3199–3209.
- Xiao-Jun H, Dan G, Zhi-Kang X. 2007. Preparation and characterization of stable chitosan nanofibrous membrane for lipase immobilization. European Polymer Journal 43(9): 3710–3718.
- Yang J, Wang F, Tan T. 2010. Degradation behavior of hydrogel based on crosslinked poly(aspartic acid). Journal of Applied Polymer Science 117: 178–185.
- Yigitoglu M, Temocin Z. 2010. Immobilization of Candida rugosa lipase on glutaraldehyde-activated polyester fiber and its application for hydrolysis of some vegetable oils. Journal of Molecular Catalysis B: Enzymatic 66: 130–135.
- Young S, Wong M, Tabata Y, Mikos A. 2005. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. Journal of Controlled Release 109(1–3): 256–274.