Abstract
Glucagon-like peptide-1 (GLP-1) as an endogenous glucose-lowering peptide has great potential in diabetes therapy, but its clinical utility is compromised by its limited activity in vivo. Herein to improve the anti-diabetic activity of GLP-1, we constructed a fusion protein (GLP-1-globular adiponectin, GAD) using this peptide and the globular adiponectin. Using recombinant expression, we prepared the GAD fusion protein with a purity of above 95%. In normal mice, we validated the acute glucose-lowering activity of GAD by performing intraperitoneal glucose tolerance test (IPGTT). After that, we evaluated the anti-diabetic activity of this fusion protein in a multiple-low-dose streptozotocin (STZ)-induced diabetic mice model. In this diabetic mice model, GAD treatment greatly reduced the elevated fasting glucose and improved their impaired glucose homeostasis as judged by oral glucose tolerance test (OGTT). After treatment, the fasting glucose was 15.34 ± 2.07 mmol/L and 9.47 ± 1.08 mmol/L for GLP-1-treated and GAD-treated diabetic mice, respectively. Moreover, GLP-1 treatment and GAD treatment improved the pancreas function of the diabetic mice by ~1.5-fold and ~4.2-fold, respectively. Furthermore, GAD treatment greatly reduced the tissue damage in the pancreas of the diabetic mice. These data suggest that GAD possesses an improved anti-diabetic activity in vivo compared with native GLP-1, and therefore it could be regarded as a potential candidate for the future development of anti-diabetic drugs.
Introduction
Along with diet and lifestyle changes, diabetes is increasing worldwide (Yang et al, Citation2010). Moreover, its associated complications are one of the major causes of morbidity and mortality (Yang et al, Citation2010). Although many kinds of anti-diabetic drugs have been developed, some diabetes patients cannot control their blood glucose using these current drugs (Nalysnyk, Hernandez-Medina, & Krishnarajah, Citation2010). Therefore, development of novel anti-diabetic drugs is essential for the treatment of diabetes and prevention of diabetes-associated complications.
Glucagon-like peptide-1 (GLP-1) is an endogenous glucose-lowering peptide (Holst, Citation2007). This peptide exerts its glucose-lowering activity through multiple pathways, including increasing insulin secretion, decreasing glucagon secretion and reducing insulin resistance (Holst, Citation2007; Baggio & Drucker, Citation2007). Moreover, studies in rodents have demonstrated that GLP-1 can induce pancreatic β-cell proliferation and reduce its apoptosis, thereby increasing the total mass of β-cells (Holst, Citation2007). Because of these advantages, GLP-1 has great potential in anti-diabetic drug development (Holst, Citation2007; Drucker & Nauck, Citation2006; Nielsen, Citation2005). However, native GLP-1 has a limited glucose-lowering activity in vivo and cannot be used for diabetes therapy directly (Drucker & Nauck, Citation2006; Nielsen, Citation2005).
To improve the anti-diabetic activity of GLP-1, many technologies have been tried before, including site-directed mutagenesis (Ma et al, Citation2010; Gao et al, Citation2010; Burcelin, Dolci, & Thorens, Citation1999), chemical modification (Veronese & Pasut, Citation2005; Gao et al, Citation2010; Youn et al, Citation2007) and fusion protein construction (Baggio, Huang, Brown, & Drucker, Citation2004; Umpierrez et al, Citation2011; Kim et al, Citation2010). In fusion protein construction, fusion partner is critical because it greatly affects the properties of the constructed fusion protein. Adiponectin is a protein secreted from the adipose tissue, and as a protein hormone it modulates multiple metabolic processes, including increasing glucose uptake, decreasing gluconeogenesis and accelerating free fatty acid oxidation (Kadowaki et al, Citation2006; Pajvani et al, Citation2003). The globular adiponectin is its truncated form and possesses all of its bioactivities.
In this study to improve the anti-diabetic activity of GLP-1, we constructed a fusion protein consisting of a mutated GLP-1 and the globular adiponectin. Using recombinant expression, we prepared the fusion protein. After that, we validated its glucose-lowering activity by performing intraperitoneal glucose tolerance test (IPGTT) in normal mice and then evaluated its anti-diabetic activity in a multiple-low-dose STZ-induced diabetic mice model.
Materials and methods
Materials
The bacterial cell strain of BL21 (DE3) was purchased from Tiangen Biotech (Beijing, China). The pET32 expression plasmid was obtained from Novagen (Darmstadt, Germany). The chromatography media of Ni-Sepharose Fast Flow and Q-Sepharose Fast Flow were purchased from Amersham-Pharmacia (Uppsala, Sweden). The male Kun Ming (KM) mice were ordered from Experimental Animal Center of Nanjing Military Region (Nanjing, China) and the use of animals has been approved by the Institutional Animal Care and Use Committee of China Pharmaceutical University. The native GLP-1 was ordered from TASH Biotech (Shanghai, China). The blood glucose determination kit was purchased from Rongsheng BioTech (Shanghai, China). The STZ was purchased from Sigma–Aldrich (St. Louis, MO).
Plasmid construction and protein purification
For fusion protein construction, the mutated GLP-1 (A2G, K28R) was put at the N-terminus and the globular domain of adiponectin at the C-terminus, with a peptide linker of alanine-methionine-glycine in the middle. We designed the DNA sequence of GAD based on its amino acid sequence and the codon bias of E. coli and then sent it out for synthesis. The synthetic gene was inserted into pET32 expression plasmid by restriction enzyme double digestion and then the constructed plasmid was confirmed by polymerase chain reaction and DNA sequencing. The bacterial cell strain of BL21 (DE3) was used as host cell for recombinant expression. For bacterial cell culture, we used an auto-induction medium (12 g/L tryptone, 24 g/L yeast extract, 30 mL/L glycerol, 1 g/L glucose, 2 g/L lactose, salts such as Na2HPO4 12.8 g/L, KH2PO4 3.4 g/L, NH4Cl 2.7 g/L, 15.8 g succinate, and Na2SO4 0.7 g/L, pH 6.8). The bacterial cell culture was conducted at 16 °C.
Bacterial cells were harvested by centrifugation at 8 000 rpm for 20 minutes, then the cell lysate was obtained through sonication disruption and the soluble proteins were isolated by centrifugation at 12 000 rpm for 20 minutes. The target protein was precipitated by 20% ammonium sulfate and then was further purified by Ni-affinity chromatography. The recombinant GAD protein was released by enterokinase cleavage and then was further purified by Ni-affinity chromatography and ion-exchange chromatography. The protein purity was determined by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and evaluated by Bio-Rad Quantity One 1-D software.
IPGTT in normal mice
The KM mice were adapted in standard conditions for 3 days and then were divided into two groups (n = 8 for each group): the saline group and the GAD group. Before test, the mice were first fasted overnight. Then the recombinant GAD protein (25 nmol/kg, i.p.) or saline was injected. After that, glucose was injected (2 g/kg, i.p.) and the blood samples were collected at predetermined time-points. The serums were isolated and their glucose levels were determined by a commercial glucose determination kit.
Establishment of diabetes mouse model
The male KM mice were first adapted in standard conditions for 3 days before being injected with STZ (50 mg/kg, i.p.) which was dissolved in citrate buffer (20 mmol/L, pH 4.0). The injections were performed daily for 5 days. After that the mice were kept in standard conditions for another 3 days and then fasted overnight before blood glucose determination. The mice with a fasting glucose above 11.1 mmol/L were selected and classified as diabetic mice and were used in the following studies.
Treatment of the diabetic mice
The diabetic mice were divided into three groups (n = 8 for each group), including the diabetes group, the GLP-1 group, and the GAD group. Another group of normal mice (n = 8) was added as control group. For the treated groups, GLP-1 (25 nmol/kg, i.p.) or GAD (25 nmol/kg, i.p.) was injected daily; for the diabetes group and the control group, the same volume of saline was injected. We treated the mice for 2 weeks and then kept them for another 3 days before oral glucose tolerance test (OGTT) and other determinations.
Evaluation of the treatment
The fasting glucose levels were determined at the beginning of each week throughout the treatment. For OGTT, the mice were fasted overnight and then orally administered glucose (2 g/kg). Then the blood samples were collected at predetermined time-points and their glucose levels were determined as mentioned above. The fasting insulin, triglyceride and cholesterol levels were measured by commercial kits. The homeostatic model assessment of the islet (HOMA-β) is defined as: 20 × fasting insulin/(fasting glucose‐ 3.5).
The mice were sacrificed by using carbon dioxide and then the organs of interest were dissected and stored in 10% neutral buffered formalin. Then the tissues were cut at 6 μm and stained with haematoxylin and eosin (H&E) following a standard protocol. The immunohistochemistry (IHC) staining against insulin was performed on the pancreas tissue sections. After that, the slices were examined by an optical microscope, and the pictures were taken under a magnification of 400-fold.
Statistics
The results are expressed as means ± SD. Statistical analysis was performed using Student's t-test for comparison between two groups. A value of p < 0.05 was considered as significant.
Results
Plasmid construction and protein purification
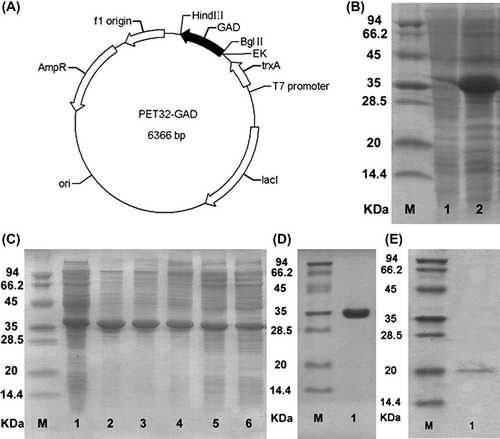
DNA sequencing showed that the expression plasmid was constructed as designed (). To improve recombinant expression, we employed auto-induction for bacterial cell culture. After auto-induction, an induced protein band appeared at ~35 kDa as expected, and its expression level was ~30% as determined by SDS-PAGE (). Using auto-induction at 16 °C, most of the expressed GAD became soluble and could be precipitated by 20% (NH4)2SO4 for preliminary purification (). To further purify this protein, we used affinity chromatography, and after that only one band could be observed in SDS-PAGE (). Then, we released GAD using enterokinase cleavage and thereafter further purified it using Ni-affinity chromatography and ion-exchange chromatography. Finally, the GAD recombinant protein was obtained with a purity of above 95% as determined by SDS-PAGE ().
Figure 1. Recombinant expression of GAD. (A) Plasmid map of GAD in pET32. (B) Auto-induction-mediated recombinant expression of GAD. M, protein Marker; 1, total protein before auto-induction; 2, total protein after auto-induction. (C) Ammonium sulfate precipitation of GAD. M, protein maker; 1, supernatant of cell lysate; lane 2 to 6 are the samples from ammonium sulfate precipitation (20%– 60% ammonium sulfate). (D) Purified Trx-GAD. M, protein marker; 1, purified Trx-GAD. (E) Purified GAD. M, protein marker; 1, purified GAD.
IPGTT in normal mice
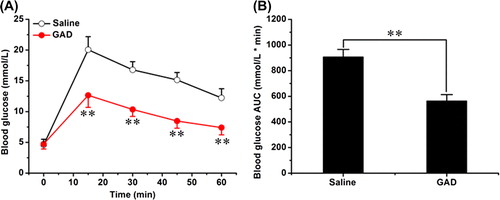
After obtaining the recombinant fusion protein, we confirmed its glucose-lowering activity in normal mice by performing IPGTT. In the tolerance test, GAD markedly reduced glucose excursion (). The glucose peak levels were 20.06 ± 2.12 mmol/L and 12.65 ± 1.97 mmol/L for saline-treated mice and GAD-treated mice, respectively. The area under curve (AUC) was 906.13 ± 59.20 mmol/L min for saline-treated mice and 562.81 ± 50.19 mmol/L min for GAD-treated mice; GAD treatment reduced the AUC by ~38% (). These data demonstrate that GAD possesses an acute glucose-lowering activity.
Fasting glucose of the treated mice
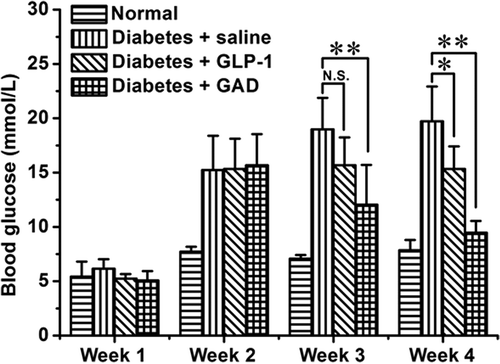
The multiple-low-dose STZ-induced diabetic mice model is well documented and has been widely used (McEvoy, Andersson, Sandler, & Hellerstrom, Citation1984). In this diabetic mice model, GAD treatment greatly reduced the elevated fasting glucose (). After treatment for 2 weeks, the fasting glucose was 19.72 ± 3.21 mmol/L for saline-treated mice, 15.34 ± 2.07 mmol/L for GLP-1-treated mice and 9.47 ± 1.08 mmol/L for GAD-treated mice (). The GAD-treated diabetic mice had a significantly reduced fasting glucose which was similar to that of normal mice, suggesting that their impaired glucose homeostasis had been greatly improved by GAD treatment.
OGTT in diabetic mice
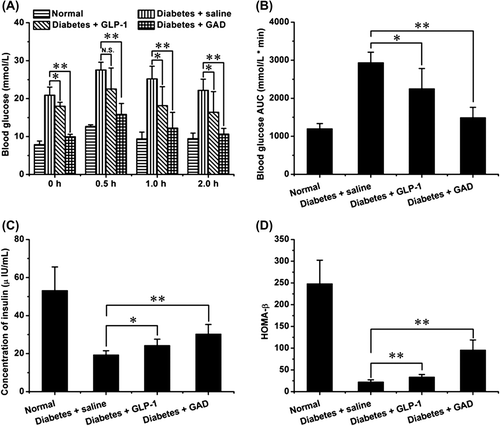
To further investigate the impact of GAD treatment on glucose homeostasis, we performed OGTT in these treated diabetic mice. In OGTT, GAD treatment markedly improved the glucose profile. The glucose peak level was 27.56 ± 2.01 mmol/L for diabetic mice treated with saline, 22.53 ± 5.54 mmol/L for diabetic mice treated with GLP-1 and 15.82 ± 2.89 mmol/L for diabetic mice treated with GAD (); GLP-1 treatment and GAD treatment reduced the AUC of glucose profile by 23% and 49%, respectively (). The improved glucose profile in the tolerance test suggested that the glucose intolerant status of the diabetic mice has been significantly improved by the GAD treatment.
Insulin level and HOMA-β
We also determined the fasting insulin levels in the diabetic mice after treatment and found that the GAD-treated mice had a higher level of fasting insulin compared with the diabetic mice (). However, the fasting insulin of the GAD-treated mice was still significantly lower compared with that of normal mice, indicating that the pancreas function of the GAD-treated mice were only partially restored. This speculation was further confirmed by HOMA-β evaluation which is a parameter for pancreas function. The HOMA-β of the diabetic mice was increased ~1.5-fold by GLP-1 treatment and ~4.2-fold by GAD treatment; however, both of them are still significantly lower than that of normal mice ().
H&E staining and IHC staining
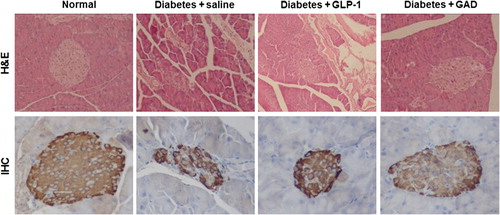
To explore the impact of GAD treatment on the pancreas, we performed H&E staining and IHC staining on these tissue sections. Injections of multiple-low-dose STZ caused severe tissue damage in the pancreas as judged by H&E staining and IHC staining (). GAD treatment greatly reduced the tissue damage in the pancreas and partially restored its insulin level (), indicating that GAD treatment had a direct beneficial effect in the pancreas.
Discussion
In this study, we constructed a fusion protein using GLP-1 and globular adiponectin and then evaluated its anti- diabetic activity in a multiple-low-dose STZ-induced diabetic mice model. In normal mice, we showed that GAD possesses an acute glucose-lowering activity. In diabetic mice, we demonstrated that GAD has an improved anti-diabetic activity as judged by fasting glucose, OGTT, insulin level and the results of histochemistry.
We designed the fusion protein based on the molecular properties of GLP-1 and globular adiponectin. GLP-1 is a glucose-lowering peptide which has been extensively studied (Holst, Citation2007). Its active site is located at the N-terminus, especially the first few amino acids (Adelhorst, Hedegaard, Knudsen, & Kirk, Citation1994), and modification at the N-terminus could markedly reduce or even deprive its glucose-lowering activity (Youn et al, Citation2007; Siegel et al, Citation1999). Therefore, we placed GLP-1 at the N-terminus of the fusion protein. Considering its stability, we used a mutated GLP-1 (A2G, K28R) but not native GLP-1 for GAD construction. Adiponectin plays an important role in glucose homeostasis and energy metabolism, and its structure-activity relationship has been well characterised (Kadowaki et al, Citation2006; Pajvani et al, Citation2003). The C-terminal globular domain is its active centre and possesses all of its bioactivities (Pajvani et al, Citation2003). Therefore, we employed this globular domain but not the full-length protein for GAD construction.
A proper linker is essential for fusion protein construction; however, a linker may also raise the risk to induce immunogenicity, antigenicity and even toxicity (Zhao et al, Citation2008). Therefore, the linker structure is important for fusion protein construction. In this study, we employed a short peptide linker (alanine-methionine-glycine) for GAD construction to reduce the risks mentioned above. Constructed using this short linker, the GAD recombinant protein maintained its acute glucose-lowering activity in normal mice and displayed an improved anti-diabetic activity in diabetic mice without causing unwanted side effects.
GAD therapy partially repaired the STZ-induced histological impairment in the pancreas, and this repairing effect may be mainly derived from its activity as a GLP-1R agonist. In rodents, it has been demonstrated that GLP-1 activation can induce pancreatic β-cell proliferation and reduce its apoptosis, thereby increasing the total mass of β-cells (Rolin et al, Citation2002; Xu, Stoffers, Habener, & Bonner-Weir, Citation1999). In this study, GAD treatment markedly reversed the STZ-induced tissue damage in the pancreas as judged by H&E staining and IHC staining, supporting these previous studies. Moreover, GAD treatment restored the insulin levels both in the pancreatic β-cells and in the circulation, further demonstrating that GAD treatment not only repaired the tissue damage but also improved its function, and this could also be judged by the improved glucose tolerance after treatment.
In conclusion, in this study we constructed a fusion protein and demonstrated that this fusion protein possesses an improved anti-diabetic activity compared with the native GLP-1. Therefore, this fusion protein could be regarded as a potential candidate for the future development of anti-diabetic drugs.
Acknowledgements
This research was supported by the China National Nature Science Foundation (30973667, 81172974), Jiangsu Province “Qing Lan Project” (2010), and the Perspective Research Foundation of Production Study and Research Alliance of Jiangsu Province of China (BY2011159).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adelhorst K, Hedegaard BB, Knudsen LB, Kirk O. 1994. Structure-activity studies of glucagon-like peptide-1. J Biol Chem. 269:6275–6278.
- Baggio LL, Drucker DJ. 2007. Biology of incretins: GLP-1 and GIP. Gastroenterology. 132:2131–2157.
- Baggio LL, Huang Q, Brown TJ, Drucker DJ. 2004. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes. 53:2492–2500.
- Burcelin R, Dolci W, Thorens B. 1999. Long-lasting antidiabetic effect of a dipeptidyl peptidase IV-resistant analog of glucagon-like peptide-1. Metabolism. 48:252–258.
- Drucker DJ, Nauck MA. 2006. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 368:1696–1705.
- Gao M, Ma C, Liu WC, Zhu J, Tian H, Gao XD, Yao W. 2010. Production and purification of an analog of glucagon-like peptide-1 by auto-induction and on-column cleavage in Escherichia coli. World J Microbiol Biotechnol. 26:1675–1682.
- Gao M, Tian H, Ma C, Gao X, Guo W, Yao W. 2010. Expression, purification, and C-terminal site-specific PEGylation of cysteine-mutated glucagon-like peptide-1. Appl Biochem Biotechnol. 162:155–165.
- Holst JJ. 2007. The physiology of glucagon-like peptide 1. Physiol Rev. 87:1409–1439.
- Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. 2006. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 116:1784–1792.
- Kim BJ, Zhou J, Martin B, Carlson OD, Maudsley S, Greig NH, Mattson MP, et al. 2010. Transferrin fusion technology: A novel approach to prolonging biological half-life of insulinotropic peptides. J Pharmacol Exp Ther. 334:682–692.
- Ma C, Gao M, Liu W, Zhu J, Tian H, Gao X, Yao W. 2010. Intein-mediated expression and purification of an analog of glucagon-like peptide-1 in Escherichia coli. Protein Pept Lett. 17:1245–1250.
- McEvoy RC, Andersson J, Sandler S, Hellerstrom C. 1984. Multiple low-dose streptozotocin-induced diabetes in the mouse. Evidence for stimulation of a cytotoxic cellular immune response against an insulin-producing beta cell line. J Clin Invest. 74:715–722.
- Nalysnyk L, Hernandez-Medina M, Krishnarajah G. 2010. Glycaemic variability and complications in patients with diabetes mellitus: evidence from a systematic review of the literature. Diabetes Obes Metab. 12:288–298.
- Nielsen LL. 2005. Incretin mimetics and DPP-IV inhibitors for the treatment of type 2 diabetes. Drug Discov Today. 10:703–710.
- Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, et al. 2003. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem. 278:9073–9085.
- Rolin B, Larsen MO, Gotfredsen CF, Deacon CF, Carr RD, Wilken M, Knudsen LB. 2002. The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases beta-cell mass in diabetic mice. Am J Physiol Endocrinol Metab. 283:E745–2013;E752.
- Siegel EG, Gallwitz B, Scharf G, Mentlein R, Morys-Wortmann C, Folsch UR, Schrezenmeir J, et al. 1999. Biological activity of GLP-1-analogues with N-terminal modifications. Regul Pept. 79:93–102.
- Umpierrez GE, Blevins T, Rosenstock J, Cheng C, Anderson JH, Bastyr EJ III. 2011. The effects of LY2189265, a long-acting glucagon-like peptide-1 analogue, in a randomized, placebo-controlled, double-blind study of overweight/obese patients with type 2 diabetes: the EGO study. Diabetes Obes Metab. 13:418–425.
- Veronese FM, Pasut G. 2005. PEGylation, successful approach to drug delivery. Drug Discov Today. 10:1451–1458.
- Xu G, Stoffers DA, Habener JF, Bonner-Weir S. 1999. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 48:2270–2276.
- Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, Shan Z, et al. 2010. Prevalence of diabetes among men and women in China. N Engl J Med. 362:1090–1101.
- Youn YS, Chae SY, Lee S, Jeon JE, Shin HG, Lee KC. 2007. Evaluation of therapeutic potentials of site-specific PEGylated glucagon-like peptide-1 isomers as a type 2 anti-diabetic treatment: insulinotropic activity, glucose-stabilizing capability, and proteolytic stability. Biochem Pharmacol. 73:84–93.
- ZhaoHL, YaoXQ, XueC, WangY, XiongXH, LiuZM. 2008. Increasing the homogeneity, stability and activity of human serum albumin and interferon-alpha2b fusion protein by linker engineering. Protein Expr Purif. 61:73–77.