Abstract
The mononuclear cells were cultivated in stirred flasks at different agitation speeds of 30 rpm, 45 rpm, 60 rpm and 80 rpm. At the agitation speed of 30 rpm, total cells achieved higher expansion folds and the CFC density increased. When at higher agitation speed of 60 rpm or 80 rpm, the number of cells dropped rapidly and characteristics of hematopoietic stem/progenitor cells (HSPCs) were not maintained. Moreover, the culture duration of 6–9 days was better for HSPCs ex vivo expansion. These data indicated that HSPCs should be cultured at relatively low agitation speed and for a short-term period when cultured in stirred suspension system.
Introduction
Since the first cord blood transplantation to treat fanconi anemia was carried out in 1988 (CitationGluckman, Broxemeyer, & Auerbach, 1989), clinical applications of cord blood stem cell transplantation has become available in the treatment of severe diseases such as blood cancer, aplastic anemia, immune deficiency disease and genetic diseases (CitationCairo & Wagner, 1997). However, one of the major difficulties in cord blood transplantation is that the number of hematopoietic stem/progenitor cells obtained from one cord blood sample is always not sufficient to engraft patients over 50 kg (CitationBornstein et al., 2005; CitationDahlberg, Delaney, & Bernstein, 2011). Therefore, it becomes necessary for ex vivo expansion of hematopoietic stem/progenitor cells before transplantation.
In general, there are two culture methods to expand cord blood hematopoietic stem/progenitor cells ex vivo including static culture and dynamic culture (CitationLiu, Liu, Fan, Ma, & Cui, 2006; CitationKing & Miller, 2007). However, the static culture has many disadvantages such as prone to form unfavorable concentration gradient, difficult for scale up, labor-intensive, and unable for on-line monitoring. Dynamic culture in bioreactor can overcome the above shortcomings of static culture method (CitationCollins, Miller, & Papoutsakis, 1998; CitationCabral, 2001; CitationMartin, Wendt, & Heberer, 2004; CitationCabrita et al., 2003; CitationGilbertson, Sen, Behie, & Kallos, 2006). The major difference between the two types of culture system is that in the bioreactor there exists fluid flow, which generates fluid shear stress and may have certain effects on cells. Since the fluid type and characteristics are mainly determined by the rotation of the impeller in the bioreactor, to investigate the effects of rotation speed on ex vivo expanding the hematopoietic stem/progenitor cells is of great significance for the bioreactor operation and development to generate sufficient cord blood cells for clinical applications.
Materials and methods
Cell separation procedures
Cord blood was obtained from healthy donors’ umbilical vein following vaginal delivery of full term babies with consent. The isolation of mononuclear cells (MNCs) was performed within 8 hours of the acquisition of cord blood by mixing the cord blood with RPMI1640 medium at the volume ratio of 1:1, and subsequently carefully laying the mixture on the Ficoll-Histopaque (Sigma, USA) with density of 1.077 g/cm3 in 50 ml centrifuge tubes and centrifuged at 400 g for 30 min at 4 °C. MNCs were collected at the interface and washed twice with PBS. Cell counting was performed using the trypan blue exclusion method. Following these procedure, the isolated cell viability was always > 97%.
Stirred suspension culture
Cells were inoculated into 100-ml stirred flasks at 1 × 106 cells/ml with 40 ml of culture medium. Cytokines including 50 ng/ml stem cell factor (SCF), 20 ng/ml flt3-ligand (FL), 20 ng/ml thrombopoietin (TPO), 10 ng/ml interleukin-3 (IL-3) and 10 ng/ml interleukin-6 (IL-6). The agitation speed was controlled at 30 rpm, 45 rpm, 60 rpm and 80 rpm, respectively. For analysis, 5 ml of cell suspension was taken and the culture system was supplemented with 5 ml of fresh culture medium to maintain total volume.
Colony-forming cell assay
The colony forming capability of hematopoietic cells was assessed with CFC assay. Cells were plated at 1 × 104 cells/mL in 24-well plates in methylcellulose-based, semisolid cultures consisting of 0.9% methylcellulose, 30% FBS, 1% bovine serum albumin (BSA), 0.1 mM 2-mercaptoethanol, 2 mM L-glutamine, and recombinant cytokines including 50 ng/mL SCF, 20 ng/mL IL-3, 20 ng/mL GM-CSF, 20 ng/mL G-CSF and 2 U/ml EPO. The cultures were maintained at 37°C with 5% CO2 atmosphere for 14 days. Colonies formed were scored under an inverted microscope.
Flow cytometry analysis
Cells were suspended in Ethylene Diamine Tetraacetic Acid-Bovine Serum Albumin-Phosphate Buffered Saline buffer (EDTA-BSA-PBS), incubated with mouse IgG (GIBCO, USA), then reacted for 15 min with fluorescein isothiocyanate FITC-conjugated CD34 monoclonal antibodies (BD, USA) at 4 °C followed by washing with PBS twice to remove unbound antibodies. Cells were re-suspended in EDTA-BSA-PBS and subjected to two-color flow cytometric analysis. Cells labeled with FITC- mouse isotype-matched antibodies were used as negative controls. The analysis was performed with a FACS scan. At least 10 000 cells were acquired and analyzed for each sample.
Fluid flow analysis
Analysis of flow field in the culture flasks at different agitation speeds was performed with fluid dynamics analysis software CFD.
Statistical analysis
Results were presented as the mean ± standard error. Student's t-test was applied to evaluate the significance of differences. P < 0.05 was considered as significant and P < 0.01 was considered as very significant.
Results
Fluid flow analysis at different agitation speeds
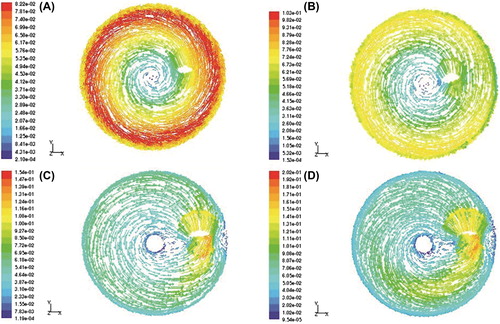
Analysis of flow field in the culture flasks at different agitation speeds was performed with fluid dynamics analysis software CFD, the results were shown in . It could be observed from that the fluid with relatively high velocity under agitation at 30 rpm () and 45 rpm () was mainly concentrated in the region near the flask wall, while the fluid velocity was relatively low near the impeller. These results indicated that the fluid shear stress was higher around the bottle wall than that close to impeller. In contrast, at 60 rpm () and 80 rpm () of agitation, the highest flow rate was distributed around the impeller and the flow rate around the flask wall was relatively small. Therefore, with high agitation speed the fluid shear stress was larger around the impeller. Furthermore, the fluid flow rate at higher agitation speed tended to be more homogenous in distribution.
Total cell expansion at different agitation speeds
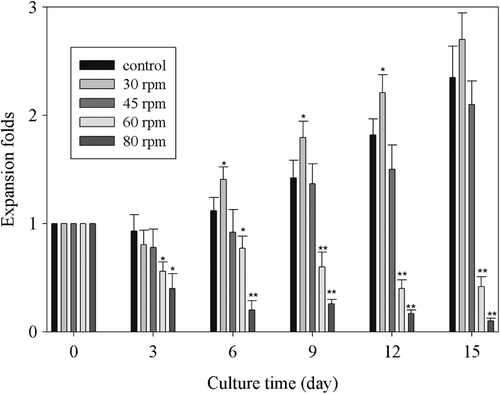
Cells were cultivated in the stirred flasks with the agitation of 30 rpm, 45 rpm, 60 rpm and 80 rpm, respectively. The expansion fold of total cells and the growth curve were shown in . The cells agitated at 30 rpm demonstrated higher expansion fold than the control group from day 6 to day 12 of the culture. And the total cell expansion was similar to the control group when agitated at 45 rpm. However, if the agitation speed was increased to 60 rpm or 80 rpm, the total cell expansion was significantly less than the control group from day 3. Therefore the optimal agitation would do favor to regulate the homogeneity of the culture environment to expand cells effectively.
CFC density in culture at different agitation speeds
Colony forming cell (CFC) density in culture reflects the expansion of hematopoietic stem/progenitor cells. The colony forming capacity of the expended cells under different agitating speeds was studied. The result was summarized in . The CFC density in culture of 30 rpm group was 195/105 cells on day 6, which was significantly higher than that of control group (P < 0.05), while the CFC densities of these two groups got close on day 9. When the agitation speed was 45 rpm, the CFC density in culture was as much as that of control group on day 6 and significantly lower than that of control group on day 9 (P < 0.01). In 60 rpm and 80 rpm groups the CFC density was only 47/105 cells and 13/105 cells on day 3, respectively, which showed significant difference to that of control group (P < 0.01). It was obvious that lower agitation speed was beneficial to CFC expansion.
Table 1. CFC density in the culture at different agitation speeds.
CD34 + cells proportion and expansion folds at different agitation speeds
CD34 molecular is recognized as the most reliable surface marker for hematopoietic stem/progenitor cells. The disappearance of CD34 molecular on the cell surface is correlated with the stem/progenitor cell differentiating. Therefore, we further studied the effect of agitation speed on CD34 + cells expansion.
As shown in , the proportion of CD34 + cells in culture agitated at 30 rpm was similar to that of control group during the whole culture period. When the agitation speed was 45 rpm, the value was significantly lower than that of the control group (P < 0.05) on day 9. While agitated at 60 rpm and 80 rpm, the CD34 + cells population decreased sharply, which was significantly lower than that of the control group on day 6 and on day 3, respectively (P < 0.05).
Table 2. CD34 + cell population in the culture at different agitation speeds.
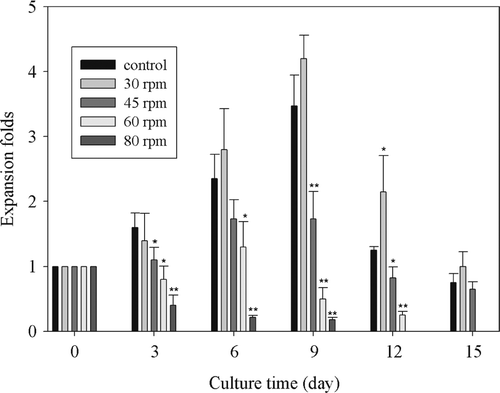
The CD34 + cells expansion at different agitation speeds were summarized in . The expansion fold of CD34 + cells agitated at 30 rpm had no significant difference from that of control group. When increased the agitation speed to 45 rpm or above, the expansion fold of CD34 + cells were significantly lower than that of the control group. It indicated that it was harmful to the cell proliferation when increased the agitation speed.
Discussion
Umbilical cord blood is rich in hematopoietic stem/ progenitor cells than bone marrow and peripheral blood. Furthermore, it is easy to harvest, and cells isolated from umbilical cord blood have high expansion potential and low immunological reaction. Therefore, it is considered as an alternative of bone marrow transplants (CitationRubinstein, Carrier, & Scaradavou, 1998; CitationFasouliotis & Schenker, 2000; CitationGoussetis et al., 2010).
Current ex vivo expansion culture methods included static culture method and dynamic culture method. In static culture, cells normally deposit at the bottom of culture plates, which generates oxygen and cytokine concentration gradients, which can negatively affect both cell proliferation and phenotype (CitationCabrita et al., 2003). However, in dynamic culture system such as stirred flasks or bioreactor, there exist different types of fluid flow including laminar flow, turbulence flow, and swirl flow. The fluid shear stress can directly act as mechanical signals acting on cells. Yang et al. demonstrated that static cultures favored the expansion of HSPCs and stirred cultures were more effective in preserving functional HSCs, so that static culture and stirred culture may be combined to guarantee both the quantity and quality of HSCs, providing helpful clues for developing novel culture systems (CitationYang, Cai, & Tan, 2008). Different bioreactors have been developed for cultivating hematopoietic stem/progenitor cells (CitationKoller, Manchel, Newsom, Palsson, & Palsson, 1995; CitationHorner, Miller, Ottino, & Papoutsakis, 1998; CitationHighfill, Haley, & Kompala, 1996). In these bioreactors, different agitation speeds can generate different fluid environments, and thus apply different degrees of fluid shear stress to cells. It is clear that there is an optimal shear stress range for cell aggregate and embryoid body cultures, with extensive clumping at very low shear stress and cell death at high stress (CitationKallos, Behie, & Vescovi, 1999; CitationJelinek et al., 2002; CitationYoun, Sen, Behie, Girgis, & Hassell, 2006; CitationYoun et al., 2005; CitationCormier, Nieden, Rancourt, & Kallos, 2006; CitationFok & Zandstra, 2005; CitationSchroeder et al., 2005).
Our study showed that in ex vivo dynamic suspension culture of hematopoietic stem/progenitor cells, agitation speeds played a critical role in their expansion and phenotype. The cells should be cultivated at a relatively low agitation speed since high agitation speed could induce cell death and differentiation. When cultured in the stirred flasks at relatively high agitation speed (60 rpm and 80 rpm), the cells number dropped rapidly and characteristics of hematopoietic stem/progenitor cells were not well maintained. When cultured at relatively low agitation speed (30 rpm), the cells achieved higher expansion folds. Furthermore, cells cultivated with agitation at 30 rpm had highest CFC density. Only on this condition can the cells not only get enough expansion folds but also have better quality. In addition, the blade shape of stirred bioreactors also plays an important role, which can generate different fluid shear stress to cells. In our study, the blade was a glass rod which can generate relatively lower fluid shear stress to the cells than the flabellum blade. However, how various types of fluid shear stress generated in the stirred bioreactor will influence the gene expression of hematopoietic stem/progenitor cells and their differentiation potential still waits to be investigated.
Moreover, the culture time was also a very important parameter since long-term culture led to cell depletion and loss of function. However, if the culture time is too short (less than 6 days), the quantity of cells might not be sufficient, and if cultivation lasts too long (beyond 9 days), cell differentiation became significant and these cells may eventually lose clinical utility. Therefore a 6 to 9-day culture period might be optimal, in which hematopoietic stem/progenitor cells could amplify most and also maintain relatively high colony formation capability.
Conclusions
In these experimental conditions, total cells and CFC cells achieved higher expansion folds at the agitation speed of 30 rpm when the stem cells were cultured in the stirred flasks, while high agitation speed caused significant cell loss and rapid cell differentiation. On the other hand, the culture duration of 6–9 days was better for getting higher expansion folds and maintaining the hematopoietic function in this stirred culture system at low agitation speed.
Acknowledgements
This research work was supported by the National Natural Science Foundation of China (20776043) and the Key Project of Medicine, Shanghai (074319109).
Declaration of interest
All of the authors report no declarations of interest.
References
- Bornstein R, Flores A, Montalban M, DelRey M, Serna D, Gilasanz FA (2005). A modified cord blood collection method achieves sufficient cell levels for transplantation in most adult patients. Stem Cells. 23:324–334.
- Cabral JMS (2001). Ex vivo expansion of hematopoietic stem cells in bioreactors. Biotechnol Lett. 23:741–751.
- Cabrita GJM, Ferreira BS, da Silva CL, Gonçalves R, Almeida-Porada G, Cabral JMS (2003). Hematopoietic stem cells: from the bone to the bioreactor. Trends Biotechnol. 21:233–240.
- Cairo M, Wagner J (1997). Placental and/or umbilical cord blood: an alternative source of hematopoietic stem cells for transplantation. Blood. 90:4665–4678.
- Collins P, Miller W, Papoutsakis E (1998). Stirred culture of peripheral and cord blood hematopoietic cells offers advantages over traditional static systems for clinically relevant applications. Biotechnol Bioeng. 59:534–543.
- Cormier J, Nieden N, Rancourt D, Kallos M (2006). Expansion of undifferentiated murine embryonic stem cells as aggregates in suspension culture bioreactors. Tissue Eng. 12:3233–3245.
- Dahlberg A, Delaney C, Bernstein ID (2011). Ex vivo expansion of human hematopoietic stem and progenitor cells. Blood. 117: 6083–6090.
- Fasouliotis SJ, Schenker JG (2000). Human umbilical cord blood banking and transplantation: a state of the art. Eur J Obstet Gynaecol Reprod Biol. 90:13–25.
- Fok E, Zandstra P (2005). Shear-controlled single-step mouse embryonic stem cell expansion and embryoid body-based differentiation. Stem Cells. 23:1333–1342.
- Gilbertson J, Sen A, Behie L, Kallos M (2006). Scaled-up production of mammalian neural precursor cell aggregates in computer controlled suspension bioreactors. Biotechnol Bioeng. 94:783–792.
- Gluckman E, Broxemeyer M, Auerbach A (1989). Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from a HLA-identical sibling. N Eng J Med. 32:1174–1178.
- Goussetis E, Petrakou E, Theodosaki M, Kitra V, Peristeri I, Vessalas G, Dimopoulou MN, . (2010). Directed sibling donor cord blood banking for children with β-thalassemia major in Greece: usage rate and outcome of transplantation for HLA-matched units. Blood Cells Mol Dis. 44:107–110.
- Highfill J, Haley S, Kompala D (1996). Large-scale production of murine bone marrow cells in an airlift packed bed bioreactor. Biotechnol Bioeng. 50:514–520.
- Horner M, Miller W, Ottino J, Papoutsakis E (1998). Transport in a grooved perfusion flat-bed bioreactor for cell therapy applications. Biotechnol Prog. 14:689–698.
- Jelinek N, Schmidt S, Hilbert U, Thoma S, Biselli M, Wandrey C (2002). Novel bioreactors for the cultivation of hematopoietic cells. Eng Life Sci. 2:15–18.
- Kallos M., Behie L, Vescovi A (1999). Extended serial passaging of mammalian neural stem cells in suspension bioreactors. Biotechnol Bioeng. 65:589–599.
- King JA, Miller WM (2007). Bioreactor development for stem cell expansion and controlled differentiation. Curr Opin Chem Biol. 11:394–398.
- Koller M, Manchel I, Newsom B, Palsson M, Palsson B (1995). Bioreactor expansion of human bone marrow: comparison of unprocessed, density-separated and CD 34-enriched cells. J Hematother. 4: 159–169.
- Liu Y, Liu TQ, Fan XB, Ma XH, Cui ZF (2006). Ex vivo expansion of hematopoietic stem cells derived from umbilical cord blood in rotating wall vessel. J Biotechnol. 124:592–601.
- Martin I, Wendt D, Heberer M (2004). The role of bioreactors in tissue engineering. Trends Biotechnol. 22:80–86.
- Rubinstein P, Carrier C, Scaradavou A (1998). Outcomes among 562 recipients of placental-blood transplant from unrelated donors. New Engl J Med. 339:1565–1577.
- Schroeder M, Niebruegge S, Werner A, Willbold E, Burg M, Ruediger M, Field LJ, . (2005). Differentiation and lineage selection of mouse embryonic stem cells in a stirred bench scale bioreactor with automated process control. Biotechnol Bioeng. 92:920–933.
- Yang S, Cai HB, Tan WS (2008). Hematopoietic reconstitution of CD34+ cells grown in static and stirred culture systems in NOD/SCID mice. Biotechnol Lett. 30:61–65.
- Youn B, Sen A, Behie L, Girgis AG, Hassell J (2006). Scale up of breast cancer stem cell aggregate cultures to suspension bioreactors. Biotechnol Prog. 22:801–810.
- Youn B, Sen A, Kallos M, Behie L, Girgis AG, Kurpios NA, Barcelon M, . (2005). Large-scale expansion of mammary epithelial stem cell aggregates in suspension bioreactors. Biotechnol Prog. 21:984–993.