Abstract
A novel N-methacryloyl-L-tryptophan methyl ester (MATrp) containing poly (hydroxyethyl methacrylate) cryogel (PHEMATrp) disc was prepared for removal of bilirubin (BR) out of human plasma. PHEMATrp cryogel disc was produced by bulk polymerization, with high gelation yield up to 92% and characterized by swelling tests, scanning electron microscopy (SEM), elemental analysis, Brunauer– Emmett–Teller (BET) analysis, contact angle measurements and surface energy calculations. BR adsorption studies were performed in a batch system, and the maximum BR adsorption capacity was found as 22.2 mg/g cryogel disc.
Introduction
BR is a highly hydrophobic compound and transported in bloodstream as a complex with albumin (water-soluble complex). In liver cells, it is conjugated to form BR glucuronides and excreted into the bile (Baydemir, Andac, Bereli, Say, & Denizli, Citation2007). Free BR is highly toxic due to its hydrophobicity. Disorders in the metabolism of BR may cause the level of free BR to increase, and it accumulates in phospholipid membranes and damages the performance of the membranes (Chou & Syu, Citation2009). Also the presence of excess BR in plasma may cause hepatitis, jaundice, brain damages or even death in newborns. Different approaches have been made to remove BR from hyperbilirubinemic human plasma for decreasing high BR level to normal, such as plasma exchange and hemoperfusion systems. Among these approaches, hemoperfusion was used to remove toxic substances from patient's plasma by passing large volumes of patient's blood over an adsorbent substance. The adsorbent's features determine the success of hemoperfusion system. The adsorbent must have the following specifications; high selectivity, good accessibility and high efficiency (Denizli, Kocakulak, & Piskin, Citation1998; Uzun & Denizli, Citation2006; Baydemir et al., Citation2009). Cryogels have been used for various applications in biotechnology and biomedical sciences. The interconnected pore morphology of the cryogels provides several advantages in downstream processes (e.g. quick swelling kinetics, low-pressure drop, short diffusion path, etc.). Macroporosity also provides short residence time during both adsorption and elution processes, so cryogels can be an alternative hemoperfusion adsorbent for removing BR from human plasma (Triphati & Kumar, Citation2011). Pseudobiospecific ligands are weak affinity ligands due to their low binding constants. Thanks to the multiple weak binding properties of the pseudobiospecific ligands, they can be used for the selective separation of the biomolecules (Pitiot, Legallais, Darnige, & Vijayalakshmi, Citation2000). Amino acids act as pseudospecific ligands, and they have key advantages over the traditional pseudospecific ligands in terms of biocompatibility, stability, low cost and high adsorption capacity. Amino acid–based functional comonomers were recently used in our previous studies (Altintaş & Denizli, Citation2009). The main advantages of this improvement are: there is no need for immobilization of the ligands to the adsorbent and no need to overcome the immobilization problems (e.g. orientation, activation, precautions), so it saves time and money.
The goal of this study is to prepare MATrp containing PHEMATrp adsorbents for BR removal from human plasma by using affinity chromatography technique. PHEMATrp cryogel disc was characterized using SEM, swelling tests, porosity measurement by BET, elemental analysis, contact angle measurements and surface free energy calculations. BR adsorption studies on PHEMATrp cryogel disc was implemented in batch system using human plasma. Elution of BR and reusability of the PHEMATrp cryogel disc were also studied.
Experimental
Materials
L-tryptophan methylester and methacryloyl chloride were supplied by Sigma (St. Louis, MO). HEMA was obtained from Sigma (St. Louise , MO), distilled under reduced pressure in the presence of hydroquinone monomethyl ether inhibitor and stored at 4°C until use. Ammonium persulfate (APS), N,N′-methylene-bis(acrylamide) (MBAAm), and N,N,N’, N’-tetramethylene diamine (TEMED) were also obtained from Sigma (St. Louise, MO). Human plasma (Cat no: P4639) and bilirubin were purchased from Sigma. All other chemicals were of reagent grade and were purchased from Merck AG (Darmstadt, Germany). Water used in the experiments was purified using a Barnstead (Dubuque, IA) ROpure LP reverse osmosis unit with a high-flow cellulose acetate disc (Barnstead D2731), followed by exposure to a Barnstead D3804 NANOpure organic/colloid removal and ion exchange packed bed system. Buffer and sample solutions were prefiltered through a 0.2-mm membrane (Sartorius, Göttingen, Germany). All glassware were extensively washed with dilute nitric acid before use.
Synthesis of MATrp
The synthesis of MATrp monomer was described previously (Altintaş & Denizli, Citation2009). First, 5.0 g of L-tryptophan methyl ester and 0.2 g of hydroquinone were dissolved in 100 mL of dichloromethane solution. After this solution was cooled down to 0°C; 12.7 g triethylamine was added and 5.0 mL of methacryloyl chloride was poured in it slowly. The final mixture was stirred magnetically for 2 h. After the chemical reaction was complete, amino acid methyl ester and triethylamine were extracted with 10% HCl. The product and methacryloyl chloride in dichloromethane were evaporated in a rotary evaporator and separated by column chromatography with chloroform/ethyl acetate mixture. The purified product (i.e. MATrp) was crystallized from ethyl acetate and cyclohexane. The 1H-NMR and 13C-NMR spectra of MATrp were used to indicate the characteristic peaks from the groups in MATrp monomer. Peaks in 1H-NMR were observed as CH3, 1.80 ppm (s); CH2, 3.42–3.36 ppm (q); CH, 4.50–4.51 ppm (m); CH2, 5.33 ppm (s); CH2, 5.64 ppm(s); NH (amid), 7.15 ppm (d); 5H (indole), 7.15–7.57 ppm (m); NH (indole), 8.25 ppm (d); and OH (acid), 11.1 ppm (s). Peaks in 13C-NMR spectrum were observed as CH3, 18.9 ppm; CH, 53.8 ppm; CH2, 65.4 ppm; C (vinyl), 110.7 ppm; CH (indole), 111.8 ppm; CH (benzene ring), 118.6 ppm; CH (benzene ring), 118.8 ppm; CH2 (vinyl), 120.0 ppm, CH (benzene ring), 121.37 ppm; CH (benzene ring), 124.0 ppm; C(indole), 127.6 ppm; C (benzene ring), 136.6 ppm; CH benzene ring, 139.9 ppm; C = O (amide), 167.9 ppm; and C = O (acid), 173.9 ppm, respectively.
Preparation of PHEMATrp cryogel disc
Preparation of PHEMATrp cryogel discs was explained in our previous studies (Yilmaz, Bereli, Yavuz, & Denizli, Citation2009). Briefly, the monomer mixture was prepared by dissolving HEMA (6 mmol) and MBAA (1 mmol) in 15 mL of deionized water with a monomer concentration of 10%. The free radical polymerization was initiated by adding APS and TEMED (1% w/v of the total monomers) in an ice bath at 0°C. Immediately, the reaction mixture was poured between two glass plates separated by 1.5 mm thick spacers. The polymerization solution between the plates was frozen at ‐16°C for 24 h and then thawed at room temperature. The resulting cryogel sheet was cut into circular pieces (0.8 cm diameter) with a perforator. PHEMA cryogel was prepared in the same manner without using MATrp monomer as a control. After washing with 200 mL of water, the cryogel discs were stored in a buffer containing 0.02% sodium azide at 4°C until use.
Characterization of PHEMATrp cryogel disc
The degree of swelling (Sw/w), the swelling ratio (%), the ratio of the total initial concentration of monomer solution (%), the cryogelation yield (%) and the macroporosity (%) of the PHEMATrp cryogel discs were determined as described in our previous work (Koç, Baydemir, Bayram, Yavuz, & Denizli, Citation2011). At least three measurements were done. The cross-sectional surface morphology of the cryogel disc was examined using SEM. The frozen cryogel sample was freeze-dried at ‐50°C using lyophilizer (Chris Alpha 1-2 LD plus, M Christ GmbH, Germany). Then, the sample was coated with gold–palladium (40:60) and examined using a JEOL JSM 5600 SEM (JEOL, JSM 5600, Tokyo, Japan). The porosity of the polymer sample was measured by the nitrogen sorption technique, performed on Flowsorb II, (Micromeritics Instrument Corporation, Norcross, USA). The specific surface area of the cryogel disc in dry state was determined by multipoint BET method (Quantachrome, Nova 2200E, USA). Elemental analysis was used to evaluate the amount of MATrp incorporated into the PHEMATrp cryogel disc, Leco Elemental Analyzer (Model CHNS-932, USA).
Contact angle studies and the surface free energy calculations were performed to evaluate the hydrophilicity/hydrophobicity of the cryogel discs. Contact angles of the PHEMA and PHEMATrp cryogel discs were measured by captive bubble method at 25°C using a digital optical contact angle meter (Kruss DSA100, Hamburg, Germany). A captive bubble needle and cuvette were filled with ultrapure water, and the sample was placed on the resting mechanisms. The curved needle was positioned under cryogel disc. At this point, a bubble of approximately 10 µL was placed under the cryogel disc surface. Contact angles (θ) for the captive bubble were measured using n-hexane (θn-hexane) and toluene (θtoluene) bubbles in water. The measurements were the average of ten contact angles at least operated on three disc samples. Using these contact angle data, the surface free energies of the discs were calculated according to Fowkes approach (CitationFowkes, 1987). By means of this arrangement, a comparison could be made between the total surface free energy of PHEMA and PHEMATrp cryogel disc samples.
The wetting of a solid surface by a liquid and the concept of contact angle (θ) were first formulated by Young's equation (1):
where γl is the surface energy of the liquid (mN/m), γsl is the interfacial energy of solid/liquid interface (mN/m) and γs is the surface energy of solid (mN/m) (Blanco, Arai, Grinberg, Yarmush, & Karger, Citation1989).
Using the Fowkes method, the polar and disperse fractions of the surface free energy of a solid can be obtained by using geometric mean approach. The resulting equation when combined with Young’s equation yields:
According to Equation 2, the polar (γsp) and disperse (γsd ) fractions of the surface free energy of the PHEMATrp cryogel were determined.
Bilirubin removal from human plasma
BR removal from human plasma was studied in a batch system. Before performing the adsorption experiments, desired amounts of BR were added into the human plasma to obtain plasma samples with the 0.1–2.5 mg/mL initial BR concentration. All adsorption experiments were performed in darkness. In a typical adsorption system, 10 mL of the BR containing human plasma was incubated with four pieces of disc sample, at 25°C for 2 h (The weight of the four pieces of dry cryogel disc is 0.34 g). The sample was withdrawn in certain time intervals in order to evaluate adsorption time parameters. After the desired treatment periods, the concentration of the BR molecules in the plasma was measured using Roche Hitachi Modular-P with Roche BR Direct, Indirect Test Kits (Diamond Diagnostics). The amount of BR adsorption per unit mass of the cryogel disc was evaluated using the following expression:
Here, Q is the amount of adsorbed BR onto unit mass of the cryogel disc (mg/g); C0 and C are the concentrations of the BR in the initial plasma and in the final plasma after treatment for a certain period of time, respectively (mg/mL); V is the volume of the solution (mL); and m is the mass of the cryogel discs used (grams).
Desorption and repeated use
The desorption of BR molecules was studied with two different desorption agents: 2 M NaOH (with EDTA, in a molar ratio of NaOH: EDTA (4:1, V:V)) and 2 M Na2CO3 solution, respectively. The PHEMATrp membranes were placed in this desorption medium and stirred continuously (at a stirring rate of 400 rpm) at room temperature for 2 h. The final BR concentration in desorption medium was measured by Roche Hitachi Modular-P, using Roche BR Direct, Indirect Test Kits. The desorption ratio for BR was calculated according to the ratio of amount of adsorbed BR cryogel disc to amount of desorbed BR concentration in the desorption medium. The reusability of the PHEMATrp discs was verified. The BR adsorption-desorption procedure was repeated ten times using the same group of discs. The PHEMATrp cryogel discs were washed with 50 mM NaOH solution to regenerate and sterilize, after each desorption step.
Results and discussion
Characterization of cryogel disc
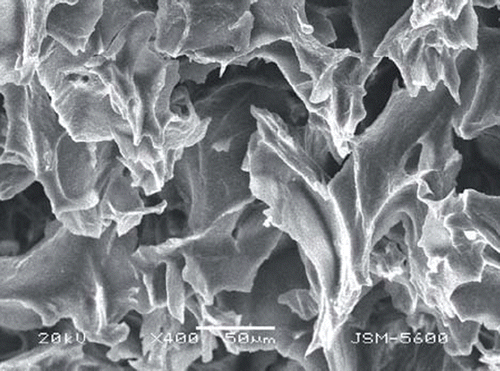
The surface morphology of PHEMATrp was exemplified by using SEM image. It obviously seems from that the internal structure of the PHEMATrp consists of large interconnected pores (10–100 µm in diameter). These supermacroporous structures of PHEMATrp discs allowed us to study highly viscous plasma samples without any clogging. The surface area and pore volume of the adsorbent affect significantly the efficiency of adsorption. According to BET analysis results, the specific surface area of the PHEMATrp cryogel disc was found as 56 m2/g.
The swelling properties of PHEMATrp cryogel discs are summarized in . The PHEMATrp cryogel discs were produced with high gelation yield (about 90%) with the swelling ratio of about 86.0 %. The incorporation of the MATrp into the PHEMATrp cryogel disc was found as 0.985 mol/g polymer by using nitrogen stoichiometry.
Table 1 Swelling properties of PHEMATrp cryogel disc.
The contact angle measurement is used for the characterization of biomaterials to describe hydrophilicity/hydrophobicity and to estimate surface free energy. A relatively small contact angle indicates a relatively more hydrophilic surface. The equilibrium contact angle values, the surface free energies and the polar and dispersive component values of the surfaces and the surface free tension values of the test liquids are summarized in . As seen in , the PHEMA cryogel disc has smaller contact angle and the surface free energy of PHEMA cryogel disc is higher than that of the PHEMATrp cryogel disc. It can be concluded that the PHEMATrp has relatively more hydrophobic surface due to the functional indole groups of PHEMATrp. Moreover, the polar fraction of the surface free energy of the PHEMATrp is lower than that of PHEMA cryogel disc. This behavior also attributed to the hydrophobic character of indole groups in PHEMATrp structure.
Table 2. Total surface energies (γ) and their dispersive (gd) and polar (gp) components determined by Fowkes approach.
Bilirubin removal studies from human plasma
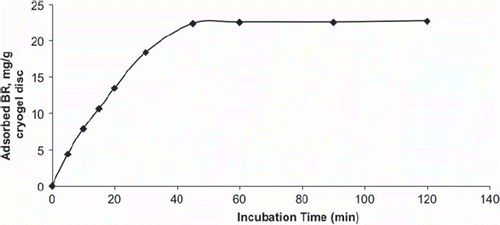
The time dependence of BR adsorption onto the PHEMATrp cryogel discs is shown in . At the beginning, the adsorption rate was relatively fast and then BR adsorption reached a plateau. The time required for achieving equilibrium conditions was about 45 min.
Figure 2. The time dependence of the adsorption values of BR on PHEMATrp cryogel disc. BR concentration: 1.0 mg/mL; mdry: 0.034 g; V:10 mL; T: 25°C; t: 2 h.
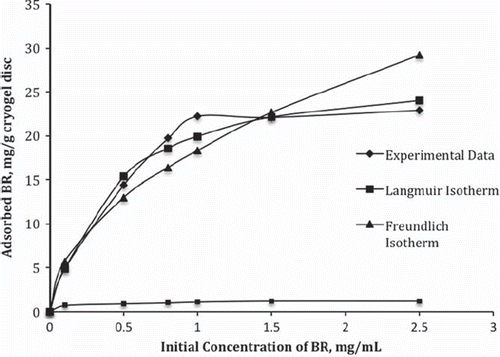
shows the effect of initial BR concentration on the BR adsorption onto the PHEMATrp cryogel disc. The amount of adsorbed BR increased with increasing BR concentration, and a saturation value was achieved at 1.0 mg/mL which represents all accessible binding sites on the PHEMATrp cryogel disc interacting with the BR molecule in plasma. Maximum adsorption capacity was found as 22.2 mg/g polymer. The results showed that the PHEMATrp cryogel discs have a higher binding capacity for BR than the control PHEMA cryogel discs 1.2 mg/g polymer; it should be keep in mind that the control cryogel discs do not include reactive functional MATrp monomer for the interaction with BR molecule.
Figure 3. Experimental data fitted with Langmuir and Freundlich adsorption isotherms. V:10 mL; mdry: 0.034 g; T: 25°C; t: 2 h.
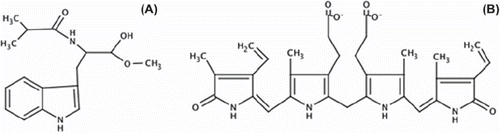
The interactions between the MATrp and the BR molecule were governed by hydrophobic interactions through the indole functional group of MATrp and pyrolle-like rings of BR (). In addition to hydrophilic groups, they exhibit hydrogen binding, van der Waals interactions and electrostatic interactions.
The Langmuir and Freundlich isotherms were calculated to understand the adsorption behavior of BR on the PHEMATrp cryogel disc. and show the comparison of the experimental adsorption behavior with Langmuir and Freundlich adsorption isotherms. As seen from , the experimental data tend to be more compatible with Langmuir instead of Freundlich isotherm. The higher the correlation coefficient (R2 = 0.99), the data is in more conformity with Langmuir adsorption isotherm than with the Freundlich isotherm. Moreover, the maximum adsorption capacity (22.3 mg/g) obtained from experimental results is very close to the calculated Langmuir adsorption capacity (24.4 mg/g). It can be concluded that favorable adsorption of BR onto the PHEMATrp cryogel disc is Langmuir monolayer adsorption model.
Table 3. Adsorption isotherm values.
Pseudo-first- and pseudo-second-order kinetic equations were used for the understanding of adsorption kinetics. Lagergren equation is a first-order rate equation that expresses the adsorption of solute from a liquid solution. First-order rate equation is represented as follows:
In Equation 4, k1 is the rate constant of the pseudo-first-order adsorption (1/min), qe is the experimental amount of BR adsorbed at equilibrium (mg/g), qt is the amount of BR adsorbed at time t (mg/g) and q1cal is the adsorption capacity calculated by the pseudo-first-order model (mg/g),
The rate constant for the second-order adsorption could be expressed as Equation 7:
where k2 is the equilibrium rate constant of pseudo-second-order adsorption (g/(mg min)) and q2cal is the adsorption capacity calculated by the pseudo-second-order kinetic model (mg/g). shows both the first-order and second-order kinetic models results. These data showed that the adsorption mechanism of BR on the PHEMATrp cryogel disc was well described by pseudo-second-order kinetic model. The rate constant for second-order kinetics (k2) is lower than pseudo-first order rate constant (k1), and the correlation coefficient value is 0.97, indicating that the adsorption rate was controlled by pseudo-second-order kinetics. It can be concluded that the overall rate of the BR adsorption process was controlled by chemical process rather than external surface mass transfer or film diffusion processes (Oncel, Uzun, Garipcan, & Denizli, Citation2005; Allen, Koumanova, Kircheva, & Nenkova, Citation2005).
Table 4. Adsorption kinetic values.
Desorption and repeated use
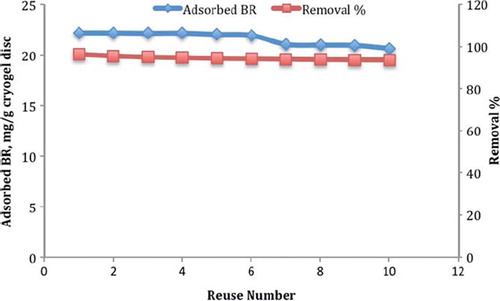
Stability and reusability of adsorbent are very important parameters for achieving minimum material consumption and decreasing the material cost (Denizli et al., Citation2003). In order to show the stability and reusability of the PHEMATrp discs, the adsorption–desorption cycle was repeated ten times using the same group discs in a batch experimental setup. For regeneration and sterilization, after one adsorption–elution cycle, the PHEMATrp discs were washed with 50 mM NaOH solution for 30 min. The removal percent decreased from 96.4% to 93.7%. As seen in , the PHEMATrp cryogel discs are very stable, and there was no remarkable decrease in the BR adsorption capacity of the cryogel discs.
Comparison with literature
In this study, BR adsorption values were found to be 22.2 mg/g cryogel disc for PHEMATrp while this value obtained was only 1.2 mg/g for PHEMA cryogel disc. Many kinds of adsorbents were reported in literature for BR removal; Kocakulak et al. presented BR adsorption capacity of 30 mg/g with their poly(glicidyl methacrylate divinylbenzene) copolymers based on albumin interactions (Kocakulak, Denizli, Rad, & Piskin, Citation1997), while Şenel et al. showed adsorption capacity of BR to be 48.9 mg/g polyamide hollow fiber based on dye ligand affinity (Senel, Denizli, Yavuz, & Denizli, Citation2002). In our previous studies, BR adsorption capacities were found as 0.85 and 3.6 mg/g polymers based on molecular recognition (Baydemir at al., 2009). Weber et al. presented adsorption capacity of BR as 17.5 mg/g with their lysine attached aluminium oxide–silica affinity membrane (Weber, Linsberger, Hauner, Leistner, & Falkenhagen, Citation2008). All these adsorbents have different structures, different functional groups and different surface areas to interact with BR molecules, as a consequence of that these adsorbents exhibit different adsorption capacities. It was seen that PHEMATrp cryogel discs have good adsorption capacity when compared with the literature.
Conclusion
In this article, we developed PHEMATrp cryogel discs as affinity matrices for removal of BR from human plasma. The PHEMATrp cryogel discs were prepared with high gelation yield, which have supermacroporous structure and high ligand content. All experiments were performed in the batch system, and BR adsorption capacity was found as 22.2 mg/g cryogel disc which is a very good adsorption capacity when compared with the related studies in the literature. Reusability performance was considerably good for these discs, so it can be concluded that PHEMATrp cryogel discs provide economic advantages. They revealed good properties as affinity discs and will be useful for removal of BR from human plasma.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Allen SJ, Koumanova B, Kircheva Z, Nenkova S. 2005. Adsorption of 2-nitrophenol by technical hydrolysis lignin-kinetics, mass transfer and equilibrium studies. Ind Eng Chem Res. 44:2281–2287.
- Altintaş EB, Denizli A. 2009. Monosize magnetic beads for lysozyme purification under magnetic field. Mat Sci Eng C. 5:1627–1634.
- Baydemir G, Andac M, Bereli N, Say R, Denizli A. 2007. Selective removal of bilirubin from human plasma with bilirubin imprinted particles. Ind Eng Chem Res. 46:2843–2852.
- Baydemir G, Bereli N, Andac M, Say R, Galaev IY, Denizli A, et al. 2009. Bilirubin recognition via molecularly imprinted supermacroporous cryogels. Coll Surf B: Biointerfaces. 68:33–38.
- Blanco R, Arai A, Grinberg N, Yarmush DM, Karger BL. 1989. Role of association on protein adsorption isotherms: β-lactoglobulin a adsorbed on a weakly hydrophobic surface. J Chromatogr A. 482: 1–12.
- Chou S, Syu M. 2009. Via zinc(II) protoporphyrin to the synthesis of poly(ZnPP-MAA-EGDMA) for the imprinting and selective binding of bilirubin. Biomaterials. 30:1255–1262.
- Denizli A, Kocakulak M, Piskin E. 1998. Bilirubin removal from human plasma in a packed-bed column system with dye-affinity microbeads. J Chromatogr B. 707:25–31.
- Denizli A, Garipcan B, Karabakan A, Say R, Emir S, Patir S, et al. 2003. Metal-complexing ligand methacryloylamidocysteine containing polymer beads for Cd(II) removal. Sep and Purif Technol. 30:3–10.
- Fowkes FM (1987). Role of acid-base interfacial bonding in adhesion. J Adhes Sci Technol. 1:7–27.
- Koç İ, Baydemir G, Bayram E, Yavuz H, Denizli A. 2011. Selective removal of 17β-estradiol with molecularly imprinted particle- embedded cryogel systems. J Hazard Mater. 192:1819–1826.
- Kocakulak M, Denizli A, Rad AY, Piskin E. 1997. New sorbent for bilirubin removal from human plasma: Cibacron Blue F3GA-immobilized poly(EGDMA-HEMA) microbeads. J Chromatogr B. 693:271–276.
- Oncel S, Uzun L, Garipcan B, Denizli A. 2005. Synthesis of phenylalanine-containing hydrophobic beads for lysozyme adsorption. Ind Eng Chem Res. 44:7049–7056.
- Pitiot O, Legallais C, Darnige L, Vijayalakshmi MA. 2000. A potential set up based on histidine hollow fiber membranes for the extracorporeal removal of human antibodies. J Membr Sci. 166:221–227.
- Senel S, Denizli F, Yavuz H, Denizli A. 2002. Bilirubin removal from human plasma by dye affinity microporous hollow fibers. Sep Sci Technol. 37:1989–2006.
- Triphati A, Kumar A. 2011. Multi-featured macroporous agarose-alginate cryogel: synthesis and characterization for bioengineering applications. Macromol Biosci. 11:22–35.
- Uzun L, Denizli A. 2006. Bilirubin removal performance of immobilized albumin in a magnetically stabilized fluidized bed. J Biomater Sci Polym Ed 17:791–806.
- Weber V, Linsberger I, Hauner M, Leistner A, Falkenhagen D. 2008. Bilirubin removal from human plasma by dye affinity microporous hollow fibers. Biomacromolecules. 9:1322–1328.
- Yilmaz F, Bereli N, Yavuz H, Denizli A. 2009. Supermacroporous hydrophobic affinity cryogels for protein chromatography. Biochem Eng J. 43:272–279.