Abstract
Inflammation is the primary problem associated with blood-contacting artificial organs. Leucocytes play an essential role in the generation of the inflammatory response. Inflammation can be defined in a variety of ways. The goal of this research is to develop a biosensor system that is less complicated and faster responding than conventional methods. In this study, highly sensitive QCM crystals were chemically modified to measure changes in adsorbed mass on the surface and were used to detect activated neutrophils. Leucocyte activation was quantified by measuring the change in frequency of the QCM. QCM crystals with immobilized anti-C3a were tested in vitro using different concentrations of neutrophils. The measured frequency shifts were proportional to neutrophil number, indicating that activated neutrophils attach to the surface of the QCM. These results were supported by AFM surface topography measurements and SEM images. This method presents a rapid, inexpensive, and easy bioassay that tests the inflammatory response to blood-contacting artificial organs.
Introduction
Currently, it is known that all biomaterials that contact the blood cause a certain amount of inflammation at the contact interface, regardless of how biocompatible the biomaterial is (Andersson et al. Citation2005, Kocakulak et al. Citation2006). However, minimal inflammation can be tolerated, which highlights the importance of the biocompatibility properties of the biomaterial.
Contact between blood and the surface of a biomaterial triggers an inflammatory response to the material. Contact between whole blood and biomaterials occurs in many different types of devices, including oxygenators, plasmapheresis, hemodialysers, catheters, stents, vascular grafts, miniature pumps, sensors, and implanted heart devices (Andersson et al. Citation2001, Rinder et al. Citation1995). The biocompatibility of artificial organs has improved rapidly in recent years (Courtney et al. Citation1994). However, problems such as inflammation, infection, or the loss of function after the procedure are still observed. For example, after cardiopulmonary bypass surgery, coagulopathies may be present and can cause serious symptoms, such as stroke. In addition, whole-body inflammatory reactions have been observed in hemodialysis patients (Nilsson et al. Citation2007).
During an inflammatory response to the surface of a biomaterial, blood coagulation or immune complement activation is observed. The activation of complement leads to the activation of leucocytes and platelets, whereas the inhibition of complement leads to a reduction in the activation platelets and leucocytes (Andersson et al. Citation2001, Nilsson et al. Citation2007, Rinder et al. Citation1995).
When a biomaterial is introduced to blood, a series of events occur. First, water is adsorbed to the surface of the biomaterial. The initial adsorption of water is followed by the adsorption of proteins. Certain proteins at the biomaterial-blood interface activate the blood coagulation system and/or the immune complement system; the activation is dependent on the type of biomaterial that is used.
Biomaterials adsorb and activate complement proteins. Complement activation can occur via three different pathways. The alternative pathway is involved in the inflammatory response to biomaterials and other foreign objects. All three of the pathways lead to the generation of the complement factors C5a and C3a. C5a is generated from C5 by C5 convertases, and C3a is generated from C3 by C3 convertases. The C5a and C3a complement factors are known as anaphylatoxins (Nilsson et al. Citation1998, Citation2007, Rinder et al. Citation1995).
Later in the inflammatory response, macrophages recognize surfaces that are coated with complement proteins, such as C3b and C3bi, as well as other proteins such as Immunoglobulin G (IgG), and migrate to the surface (Lucchesi and Mullane Citation1986). Additionally, C5a and C3a are considered to be chemotactic agents for leucocytes. C5a and C3a guide leucocytes to the site of injury or, in this case, to the surface of the biomaterial (Nimeri et al. Citation1998). Surface-induced activation of the complement system is important for acute inflammation and thus is important for biomaterials research (Nilsson et al. Citation1998, Citation2007, Rinder et al. Citation1995). For this reason, the development of different methods for screening the biocompatibility and hemocompatibility of biomaterials is needed.
The primary goal of this research is to develop a simple, inexpensive, rapid, and label-free biosensor for the detection of the complement factor C3a on the plasma membrane of activated neutrophils. To achieve this aim, a highly sensitive system for measuring the activation of complement is required. Anti-C3a was used as an affinity ligand for activated neutrophils. Piezoelectric immunosensors are viewed as attractive alternatives to conventional platforms such as flow cytometry and ELISA because QCM biosensors use label-free methods for performing assays and require less complicated procedures than conventional methods (Luppa et al. Citation2001, Pei et al. Citation1998). Therefore, we aim to develop a piezoelectric immunosensor for measuring the activation of leucocytes.
The quartz crystal microbalance (QCM) is a highly sensitive mass measurement device that operates by measuring the change in the resonance frequency of a quartz crystal by the electrodes (Kocum et al. Citation2010). The Sauerbrey equation describes the relationship between the resonance frequency and the mass on the QCM electrodes.
It is relatively simple to apply a variety of surface modifications to the QCM sensors. Sauerbrey Equation:
∆F =−2.3 × 106 F2 ∆M/A
∆F = Frequency change in the oscillating crystal in Hz,
F = Frequency of the piezoelectric quartz crystal in MHz,
ΔM = Mass of the deposited film in g,
A = surface area of the electrode in cm2.
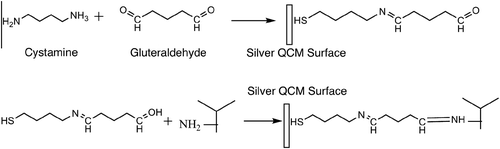
The immobilization of the antibody on the crystal is one of the most important steps in the development of QCM biosensors (Tsai and Lin, Citation2005). A variety of immobilization methods have been described, including adsorption, microencapsulation, entrapment, crosslinking, and covalent binding. In this study, cystamine is used to functionalize the surface of the QCM with amino groups (Collings and Caruso Citation1997, Zeng et al. Citation2006). These amino groups provide spacer arms for glutaraldehyde for covalent binding. Finally, anti-C3a antibodies are immobilized by binding to glutaraldehyde. In this case, the antigen is C3a (on the neutrophil), and the antibody is anti-C3a (immobilized on the surface of the piezoelectric device).
Experimental
Reagents
Anti-human C3a antibody was obtained from Becton Dickinson (N. Jersey, USA). Glutaraldehyde and cystamine were obtained from Sigma Chemical Co. (St. Louis, USA). All of the other chemicals that were used were of analytical grade.
Apparatus
Twenty QCM crystals that were 12 MHz on each side were obtained from the TIC Company and used for analysis. The electrode surface was pretreated sequentially with pure acetone, pure methanol, and 0.5 M NaOH (30 minutes for each step) to obtain a clean, silver surface (). After the pretreatment, the crystals were rinsed with deionized, distilled water in an ultrasonic washer and air-dried. These steps removed impurities from the surface of the electrode.
The frequency measurements were performed in a static system using a quartz crystal microbalance system (QCM) and a frequency counter. The measurements were performed inside an incubator to maintain a stable temperature. An experimental environment with a stable temperature and minimal flow of air is essential for the accurate measurement of the resonant frequency of QCM crystals. The resonant frequency of the crystal was measured before and after each step of the cleaning process before and after each step.
Surface modification method
The cystamine molecule has two functional groups: the SH group is called a thiol, and the NH group is called an amine (). With this property, the molecule was attached to the crystal surface by the thiol group. For the attachment reaction, a 20 mM cystamine solution was prepared by dissolving 0.4345 gr cystamine in 10 ml PBS (0.1 M, pH 7). Quartz crystals were reacted with the cystamine solution in the dark at a constant temperature; the mixture was stirred constantly with a magnetic stirrer for 30 minutes. After the reaction, the crystals were rinsed with deionized water in an ultrasonic cleaner for 4 minutes and were dried. The resonant frequency of the QCM was measured to assess the immobilization of reagents on the QCM ().
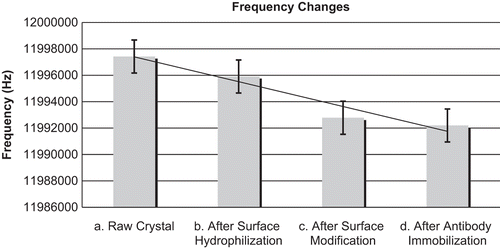
Figure 2. Average frequency changes were measured after each step of the surface cleaning and modification protocol. In each step, the frequency values decreased. (a) Base frequency values of the raw crystal after removing the metal case. (b) Frequency values after the treatment with acetone, NaOH, methanol and deionised water in the cleaning procedure. (c) Frequency values after cystamine and glutaraldehyde immobilisation. (d) Frequency values after anti-C3a immobilisation.
Glutaraldehyde was used as a spacer arm and has two functional groups. The amine groups on the cystamine molecules and aldehyde groups on the glutaraldehyde molecules were reacted and covalently bonded.
During the immobilization of ligands, the active regions of the ligand molecules participate in specific and rapid interactions with the analyte molecules. The spacer arm prevents steric interference during the interaction between the analyte and ligand molecules.
Crystals with immobilized cystamine were dipped in a tetraborate/HCl buffered (pH 8.2) 5% glutaraldehyde solution in the dark and stirred on a magnetic stirrer for 30 minutes. After the reaction, the crystals were rinsed with deionized water in an ultrasonic cleaner for 4 minutes and subsequently dried. The frequency was measured and compared to assess the immobilization ().
A 10% solution of antibody was applied to the crystals (30 µl on each side) for 30 minutes. After the incubation, the crystals were rinsed with deionized water in an ultrasonic cleaner for 4 minutes and were dried. The frequency was measured and compared to assess the immobilization.
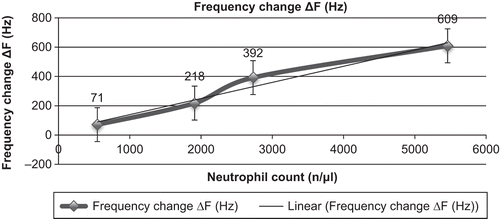
A blood sample from a healthy donor was collected, and leucocytes were prepared at 4 different concentrations for testing the biosensor in vitro. In total, four different samples were prepared to test the biosensor in vitro. To prepare diluted samples of leucocytes, blood was centrifuged at 3000 rpm, and leucocytes were collected from the buffy coat and diluted to concentrations of 50%, 35%, or 10% in PBS. The last testing sample was the whole blood sample, which was used without any treatment of the blood. After the antibody immobilization process, the crystals were dipped in the three different concentrations of the diluted leucocyte solutions or in the whole blood sample. The concentrations of the diluted leucocyte samples were 2730, 1911, and 546 neutrophils per µl, respectively. The whole blood sample had a neutrophil count of approximately 5460 per µl. After 30 minutes of stirring, the crystals were dried, and the frequency was measured ().
Results and discussion
Unmodified QCM crystals have impurities and an assortment of particles on their surfaces. After rinsing the crystals with acetone, NaOH, methanol, and deionized water, the cleaned and hydrolyzed surfaces had decreased resonant QCM frequencies compared with those that were measured before the surface cleaning.
The application of cystamine and glutaraldehyde to the surface increased the mass on the QCM, which was observed as a decreased frequency value.
Antibodies were covalently immobilized via glutaraldehyde cross-linking. Passive adsorption was used as a control. Antibodies are large molecules. Therefore, the immobilization of antibodies on the surface of the crystals resulted in a large decrease in the measured frequency.
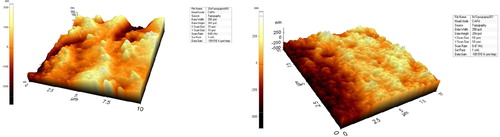
At the highest concentration of neutrophils the frequency value was much lower than that at the lowest concentration of neutrophils. This trend may occur because the activation of leukocytes is increased in samples with higher concentrations of neutrophils (). AFM measurements of these samples showed that the antibodies were immobilized on the surface ().
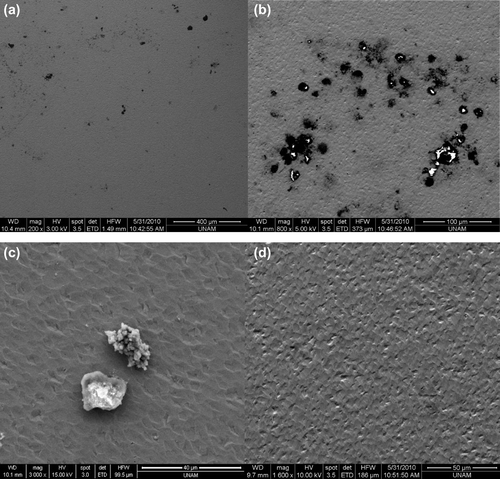
Figure 5. SEM images of the biosensors. (a) Magnified SEM image of activated neutrophils on the surface of a biosensor (200×), (b) Magnified SEM image of activated neutrophils on the surface of a biosensor (500×), (c) Magnified SEM image of activated neutrophils on the surface of a biosensor (3000×). (d) Magnified SEM image of the control group (1600×). Neutrophils were not observed on the crystal surfaces.
Conclusion
It is known that inflammatory reactions and complement activation occur at the interface between artificial organs and blood. It is important to test artificial organs for hemocompatibility, for example, by measuring the activation of complement on the surface of the artificial organ. Neutrophils play an essential role in the inflammatory response. A rapid test for neutrophil activation would be a useful tool for biocompatibility screening. The testing of the inflammatory response should be rapid, inexpensive, and easy. In this study, surface modified QCM crystals were investigated for their use in measuring the inflammatory response. The results of this study suggested the use of frequency changes in surface-modified QCM crystals to measure the inflammatory response. The results presented here were supported by AFM surface topography measurements and SEM images.
References
- Andersson J, Larsson R, Richter R, Ekdahl KN, Nilsson B. 2001.Binding of a model regulator of complement activation (RCA) to a biomaterial surface: surface-bound factor H inhibits complement activation.Biomaterials22: 2435–2443.
- Andersson J, Ekdahl KN, Lambris JD, Nilsson B. 2005.Binding of C3 fragments on top of adsorbed plasma proteins during complement activation on a model biomaterial surface.Biomaterials26: 1477–1485.
- Collings AF, Caruso F. 1997.Biosensors: recent advances.Rep Progr Phys.60: 1397–1445.
- Courtney JM, Lamba NMK, Sundaram S, Forbes CD. 1994.Biomaterials for blood-contacting applications.Biomaterials15: 737–744.
- Kocakulak M, Özgürtas T, Ayhan H. 2006.Effect of poly(2-methoxyethyl acrylate)-coated oxygenators on haemolysis.J Biomater Sci Polymer Ed.17: 449–460.
- Kocum C, Erdamar A, Ayhan H. 2010.Design of temperature controlled quartz crystal microbalance system.Instrum Sci Tech.38: 39–51.
- Lucchesi BR, Mullane KM. 1986.Leukocytes and ischemia-induced myocardial injury.Annu Rev Pharmacol Toxicol.26: 201–224.
- Luppa PB, Sokoll LJ, Chan DW. 2001.Immunosensors - principles and applications to clinical chemistry.Clin Chim Acta.314: 1–26.
- Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. 2007.The role of complement in biomaterial-induced inflammation.Mol Immunol.44: 82–94.
- Nilsson B, Larsson R, Hong J, Elgue G, Ekdahl KN, Sahu A, Lambris JD. 1998.Compstatin inhibits complement and cellular activation in whole blood in two models of extracorporeal circulation.Blood92: 1661–1667.
- Nimeri G, Fredriksson C, Elwing H, Liu L, Rodahl M, Kasemo B. 1998.Neutrophil interaction with protein-coated surfaces studied by an extended quartz crystal microbalance technique.Colloids Surf B-Biointerfaces.11: 255–264.
- Pei RJ, Hu JM, Hu Y, Zeng Y. 1998.A piezoelectric immunosensor for complement C4 using protein A oriented immobilization of antibody.J Chem Tech Biotechnol.73: 59–63.
- Rinder CS, Rinder HM, Smith BR, Fitch JCK, Smith MJ, Tracey JB, et al. 1995.Blockade of c5a and c5b-9 generation inhibits leukocyte and platelet activation during extracorporeal-circulation.J Clin Investig.96: 1564–1572.
- Tsai WC, Lin IC. 2005.Development of a piezoelectric immunosensor for the detection of alpha-fetoprotein.Sens Actuators B-Chem. 106: 455–460.
- Zeng H, Wang H, Chen FP, Xin HY, Wang GP, Xiao L, et al. 2006.Development of quartz-crystal-microbalance-based immunosensor array for clinical immuno pheno typing of acute leukemias.Anal Biochem.351: 69–76.